E¤cient GFP mutations profoundly a¡ect mRNA transcription and
translation rates
Andrea Sacchetti, Tarek El Sewedy
1
, Ashraf F. Nasr, Saverio Alberti*
Laboratory of Experimental Oncology and Biotech Group, Department of Cell Biology and Oncology,
Instituto di Ricerche Farmacologiche Mario Negri, Consorzio Mario Negri Sud, 66030 Santa Maria Imbaro (Chieti), Italy
Received 5 January 2001; accepted 6 February 2001
First published online 23 February 2001
Edited by Julio Celis
Abstract Green fluorescent protein (GFP) variants with higher
expression efficiencies have been generated by mutagenesis.
Favorable mutations often improve the folding of GFP. However,
an effect on protein folding fails to explain the efficiency of
several other GFP mutations. In this work, we demonstrate that
mutations of the GFP open reading frame and untranslated
regions profoundly affect mRNA transcription and translation
efficiencies. The removal of the GFP 5PP untranslated region
halves the transcription rate of the GFP gene, but hugely
improves its translation rate. Mutations of the GFP open reading
frame or the addition of peptide sequences differentially reduce
the GFP mRNA transcription rate, translation efficiency and
protein stability. These previously unrecognized effects are
demonstrated to be critical to the efficiency of GFP mutants.
These findings indicate the feasibility of generating more
efficient GFP variants, with optimized mRNA transcription
and translation in eukaryotic cells. ß 2001 Federation of Euro-
pean Biochemical Societies. Published by Elsevier Science B.V.
All rights reserved.
Key words: Green £uorescent protein; RNA transcription
rate; RNA translation e¤ciency; Protein stability; Protein
folding; Gene mutation
1. Introduction
Green £uorescent protein (GFP) is a spontaneously £uores-
cent protein that is widely used as a recombinant protein tag
in living cells [1^3]. However, the expression of GFP is sub-
optimal in several experimental systems [4], often because of a
low folding e¤ciency at high temperatures [4,5]. Several GFP
mutants have thus been produced that demonstrate better
£uorescence and generate higher protein levels than the
wild-type (wt) [6^11]. Quite a few of these also show an im-
proved protein folding [6,8,10,11]. However, it was open to
question if all of the mutations with major e¡ects on GFP
expression levels a¡ected protein folding or protein stability,
or if they acted through other, as yet unrecognized, mecha-
nisms [4^6,8].
Little is known about the e¡ects of structural alterations of
the GFP gene on its transcription and translation abilities
[12]. In most genes, the latter are in£uenced by the 5P and
3P untranslated regions (UTs) of the mRNA [13^18], and by
sequences surrounding the translation start site, such as the
Kozak motif [19^23]. Sequence elements in the coding region
also a¡ect the e¤ciency of transcription, the mRNA stability
and the mRNA translation rate [24^29]. Thus, we investigated
if alterations in the GFP UT and open reading frame (ORF)
(deletions, point mutations and added peptide tags, as test
sequences for fusion to heterologous proteins [30,31]) could
signi¢cantly modify the GFP mRNA transcription and trans-
lation rates, or mRNA stability. Our ¢ndings demonstrate
that mutations of the GFP ORF and UT have major conse-
quences on mRNA transcription and translation. These pre-
viously unrecognized e¡ects are demonstrated to be critical to
the e¤ciency of GFP mutants. These results indicate the fea-
sibility of more e¤cient GFP expression constructs, and of
speci¢c mutagenesis and screening programs aimed at opti-
mizing mRNA transcription and translation in eukaryotic
cells.
2. Materials and methods
2.1. Vector construction
The full-length wtGFP cDNA (p10.1 plasmid) [12] was utilized to
generate the wtGFP expression constructs with or without UTs. The
wtGFP, and the S65T [6], Bex1 and Vex1 [7,32] mutants were ampli-
¢ed by PCR and subcloned in the pRK-5 expression vector [33] (Fig.
1). N-terminal (wtGFP-myc) and C-terminal (wtGFP-SB) tagged
wtGFP were also generated. All constructs were sequenced to ascer-
tain the absence of PCR-induced mutations.
2.2. Transfection of the GFP expression constructs
293T cells were cultured in DMEM with 10% fetal calf serum
(Gibco-BRL, Paisley, UK) and 200 Wg/ml geneticin (Sigma Chemical
Co., St. Louis, MO, USA). Cells were seeded in 10 cm diameter dishes
(Nunclon, Nunc, Denmark), and transfected by calcium phosphate
co-precipitation, as previously described [34].
2.3. Northern blot analysis
RNA for Northern hybridization was extracted from transfected
293T cells. The GFP mRNA stability was quanti¢ed as described
previously [33]. Brie£y, transfected cells were treated with 10 Wg/ml
actinomycin D to stop RNA polymerase II transcription. Total RNA
was extracted at di¡erent times after the treatment and analyzed by
Northern blotting. The levels of the GFP transcripts were measured
by densitometry, and analyzed with NIH Image 1.62, using a Kodak
gray scale for internal standardization (http://www.kodak.com/coun-
try/US/en/motion/postProduction/tools/taf.shtml). mRNA levels were
plotted against time (Fig. 2), and used to determine the mRNA half-
life (Table 1). The ratio between steady-state mRNA levels and RNA
stability was used to quantify the mRNA transcription rate. Since the
mRNA stability of the di¡erent constructs was found to be essentially
identical, the levels of mRNA were used as direct indicators of
mRNA transcription rates (Table 1).
0014-5793 / 01 / $20.00 ß 2001 Federation of European Biochemical Societies. Published by Elsevier Science B.V. All rights reserved.
PII: S0014-5793(01)02246-3
*Corresponding author. Fax: (39)-0872-570 412.
E-mail: [email protected]
1
Present address: King Abdulaziz City for Science and Technology,
Riyadh, Saudi Arabia.
FEBS 24684 5-3-01
FEBS 24684 FEBS Letters 492 (2001) 151^155
brought to you by COREView metadata, citation and similar papers at core.ac.uk
provided by Elsevier - Publisher Connector
2.4. Western blot analysis
Pre-cleared cell lysates were run on 12% SDS^PAGE and trans-
ferred to nitrocellulose ¢lters. The ¢lters were treated with rabbit
anti-GFP (Clontech) and peroxidase-conjugated anti-rabbit antisera
(Calbiochem, La Jolla, CA, USA) essentially as described previously
[35]. Antibody-binding was revealed by chemiluminescence (ECL,
Amersham, Buckinghamshire, UK) (Fig. 3). The GFP protein stabil-
ity was quanti¢ed after blocking protein synthesis with cycloheximide
(25 Wg/ml). Cells were lysed at di¡erent times after the treatment, and
analyzed by Western blotting (Fig. 3). The GFP levels (P) at the
di¡erent time points were measured by densitometry, analyzed with
NIH Image 1.62, and used to determine the protein half-life (Table 1).
The mRNA translation rates of the di¡erent constructs (how many
molecules of GFP are synthesized per mRNA molecule) were
calculated as the ratio between corresponding protein and mRNA
levels. To correct for the di¡erent stabilities of the encoded pro-
teins, the protein levels were normalized versus the protein half-lives
(P/Pt
1=2
). As for the mRNA transcription rates, there was no need to
normalize for mRNA stability, since this was constant among the
di¡erent constructs (Fig. 2).
2.5. Fluorescence analysis
The GFP £uorescence analysis was performed by £ow cytometry
[34] (FACStar and Vantage, Becton-Dickinson, Synnyvale, CA, USA)
(Fig. 3), and by spectro£uorimetry (CM1T11I Spex spectro£uorime-
ter, Spex Industries, Edison, NJ, USA). Blue-excited GFPs are better
chromophores [36] than the wtGFP by 1.7-fold. However, the wtGFP
is excited about 3.5-fold less at 488 nm than at 396 nm [37,38]. Thus,
blue-excited GFPs emit around 6-fold more light than wtGFP when
excited at 488 nm in £ow cytometry. Fluorescence half-lives essentially
coincided with those of the corresponding peptides reported in Table
1.
3. Results and discussion
The wtGFP, and ¢rst (S65T) [6] and second (Bex1 and
Vex1) [7] generation GFP mutants were expressed in mamma-
lian cells and analyzed. These constructs were designed to
include either or both the 5P and 3P UTs of the GFP gene,
N- or C-terminal GFP amino acidic tags, di¡erent Kozak
initiation of translation sequences [21], and di¡erent muta-
tions of the GFP ORF (Fig. 1 and Table 1). The S65T con-
struct bears this single mutation [6], whereas Bex1 and Vex1
bear multiple ORF mutations (S65T, V163A for Bex1;
V163A, S202F, T203Y for Vex1) [7,32]. All constructs were
transiently transfected into mammalian cells. The GFP
mRNA transcription and translation rates and stabilities
were measured, together with the GFP protein and £uores-
cence levels and stabilities (Figs. 2 and 3). The ratio between
steady-state mRNA levels and RNA stability was used to
quantify the GFP mRNA transcription rates. Since the
mRNA stability was found constant for the di¡erent GFP
variants, the mRNA levels were used as direct indicators of
the e¤ciency of mRNA transcription (Table 1). The ratio
between steady-state protein and mRNA levels was used to
quantify the mRNA translation rates. This ratio was normal-
ized versus the protein half-life, to correct for the di¡erential
e¡ects of each mutation on protein stability. Table 1 presents
the results obtained in transfected 293T cells. Analogous re-
sults were obtained in transfected COS-7 and 293 cells, sup-
porting the widespread validity of these ¢ndings.
3.1. UTs
The deletion of the 5P UT from wtGFP caused a 50% re-
duction in the GFP mRNA levels (Table 1). Since the stability
of the v5P UT mRNA was not a¡ected (Table 1 and Fig. 2),
this indicated that the removal of the 5P region of the gene
reduced the GFP mRNA transcription rate by 50%. On the
other hand, the deletion of the 5P UT led to a 200-fold in-
crease in the GFP protein levels (Table 1 and Fig. 3; wtGFP-
v5P UT versus wtGFP) [12]. The wtGFP-v5P UT and wtGFP
constructs expressed identical, native GFP proteins, and these
were con¢rmed to have an identical half-life (Table 1). Thus,
wtGFP-v5P UT mRNA possesses a 200-fold higher transla-
tion rate than wtGFP mRNA. The wtGFP-v5P UT and
wtGFP share identical Kozak initiation of translation sequen-
ces (Table 1 and see below) [21]. Thus, potent translation
regulatory elements other than the Kozak motif exist within
the 5P UT of GFP. These may impair ribosome scanning [39]
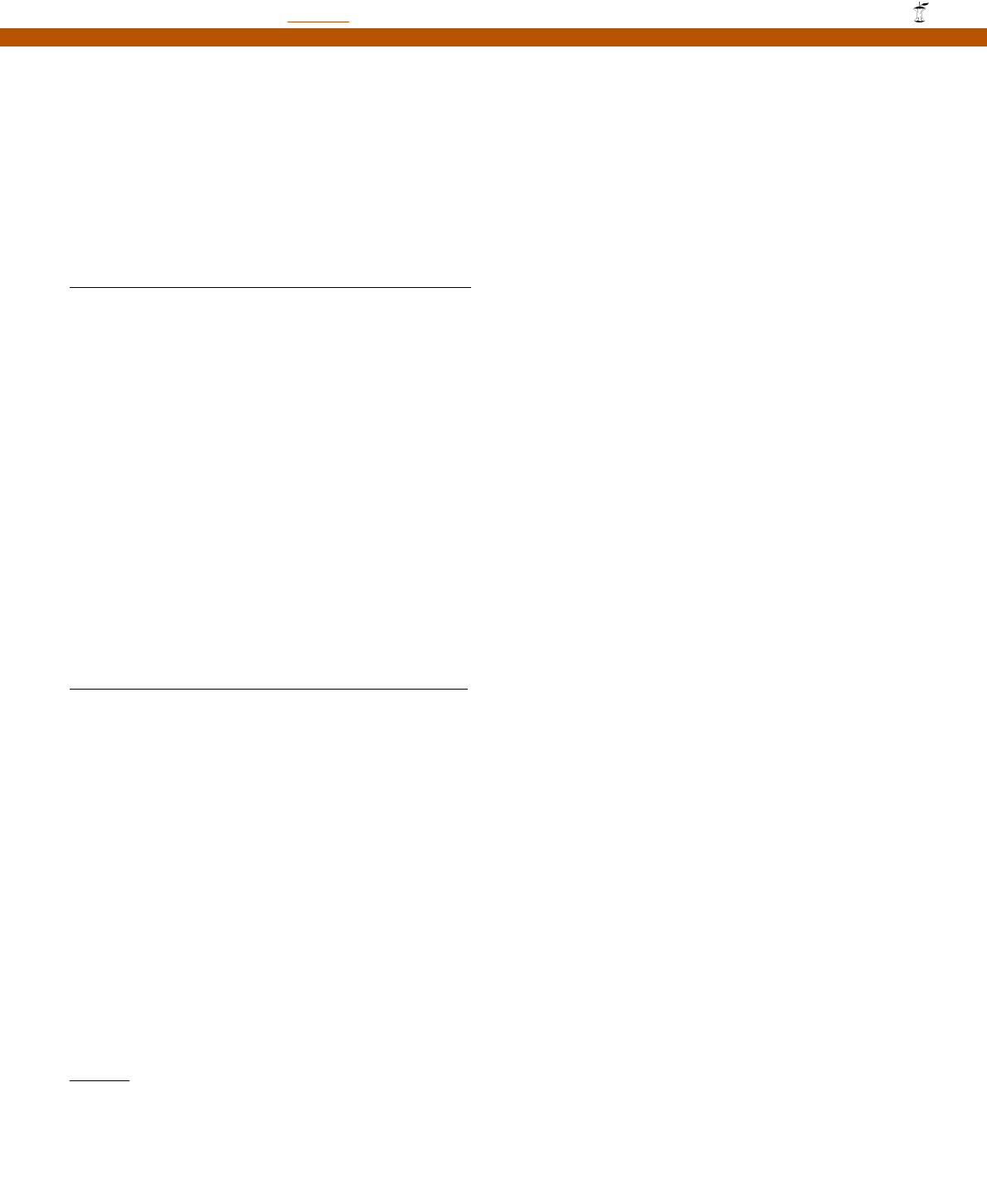
Fig. 1. Engineered GFP variants. wtGFP: full-length wtGFP cDNA in germline con¢guration. This includes the 5P and 3P UTs. wtGFP-v5P
UT: wtGFP that includes the 3P UT, but is devoid of the 5P UT; GFP-myc: N-terminal myc-tagged GFPs (wt, S65T, Vex1 or Bex1), devoid
of the 5P and 3P UTs; GFP-SB: C-terminal streptavidin-binding tagged wtGFP, devoid of the 5P and 3P UTs. UT: untranslated region; bent
arrow: ATG translation start codon; stop: stop codon; MYC : myc tag; SB: streptavidin-binding tag.
FEBS 24684 5-3-01
A. Sacchetti et al./FEBS Letters 492 (2001) 151^155152
or bind negative modulators of GFP translation [29,40], and
their removal allows the e¤cient translation of the GFP
mRNA. On the other hand, all of the mRNA devoid of a
3P UT displayed a lower translation rate than the wtGFP-
v5P UT, suggesting a translation-enhancing e¡ect of the 3P
UT of GFP [16,18].
3.2. Point mutations
Mutations in the GFP ORF were found to a¡ect the trans-
lation rate of the corresponding mRNA. The V163A mutation
was shown to cause a reduction in the translation rate of the
GFP mRNA by almost one-half (Table 1: compare Bex1-myc
with S65T-myc). This result was rather unexpected, since
Bex1-myc is an e¤cient V163A mutant [7]. However, a sim-
ilarly low translation rate was demonstrated by Vex1-myc
(Table 1). The ¢nding that a single codon change in the
GFP ORF is su¤cient to profoundly a¡ect the GFP trans-
lation rate is novel. It would be of interest to determine if this
single codon mutation directly a¡ects translation, e.g. by
translation pausing due to RNA secondary structures [28],
or if it induces the binding of translation-inhibitory factors
[29].
3.3. GFP tags
GFP is routinely used as a fusion partner to heterologous
proteins, and these have been demonstrated to heavily a¡ect
GFP function [5,30,41]. GFP tags known to a¡ect the folding
of GFP [41] were used in this work as examples of heterolo-
gous fusion partners. Remarkably, the SB tag was found to
a¡ect both mRNA transcription and translation rates, con-
¢rming the role of coding sequences in regulating the mRNA
transcription e¤ciencies [24] and translation rates [28,29].
The wtGFP-SB and wtGFP-myc bear Kozak initiation of
translation sequences of di¡erent strength [21] (Table 1).
However, di¡erent Kozak sequences are unlikely to account
for major di¡erences in GFP translation e¤ciencies. Indeed,
wtGFP-v5P UT demonstrates the highest translation rate
among the constructs tested in this work. In particular, it is
twice as e¤cient as wtGFP-myc, in spite of the largely sub-
optimal Kozak sequence of the former versus the `perfect'
Kozak motif in the latter [21,42]. The 200-fold better trans-
lation of wtGFP-v5P UT compared to that of the wtGFP, in
spite of an identical germline Kozak motif, is also consistent
with an overall minor role in GFP translation (Table 1, Fig. 3
and see above). Kozak motifs were ¢rst identi¢ed by compar-
ison of numerous eukaryotic mRNA sequences that surround
the AUG start codon [43]. However, the relative strength of
di¡erent Kozak sequences was experimentally assessed largely
in expression systems for preproinsulin or chloramphenicol
acetyl transferase [21]. Thus the strength of Kozak sequences
may vary depending on their sequence context [44]. In other
words, mRNA elements other than the Kozak motif contrib-
ute to the determination of their overall translation compe-
tence, and the relative importance of these di¡erent elements
probably varies among di¡erent mRNAs.
wtGFP-SB, Bex1-myc and Vex1-myc demonstrate the high-
est folding levels (up to 80% of the GFP molecules) [41] and
the lowest translation rates among the GFP variants analyzed
(Table 1). This raised the possibility that high folding levels
were mechanistically linked to a `slow' translation [45,46].
However, a comparison of wtGFP and wtGFP-v5P UT, which
are identical wtGFP molecules with widely di¡erent mRNA
translation rates, demonstrates essentially identical protein
folding abilities (25% of the GFP molecules [41]). Thus, the
GFP folding e¤ciency appears to be a fundamental property
of speci¢c peptide sequences, and is unlikely to be causally
related to translational `speed'.
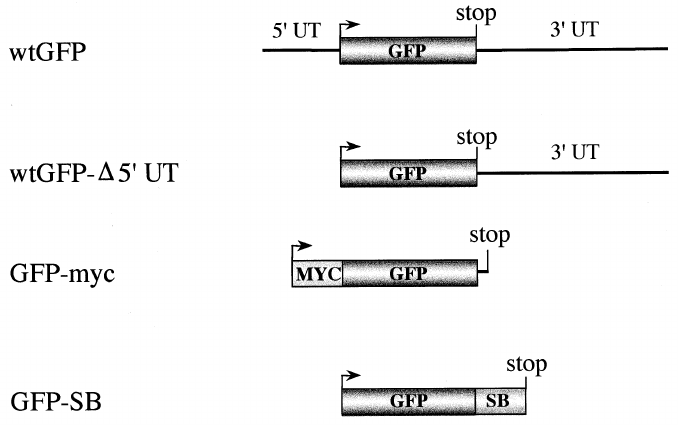
Fig. 2. GFP mRNA stability in transfected 293T cells. mRNA syn-
thesis was blocked with actinomycin D and the relative residual
amounts of GFP mRNA were quanti¢ed at di¡erent time points.
wtGFP: squares ; wtGFP-v5P UT: triangles; wtGFP-myc: circles.
Table 1
GFP mRNA stabilities and transcription/translation rates
GFP form mRNA levels
a
mRNA t
b
1=2
P
c
Pt
d
1=2
Translation rate
e
Kozak sequence
f
wtGFP 100 7 1 54 1 caaagATGa
wtGFP-v5P UT 50 7 170 54 210 caaagATGa
wtGFP-SB 80 30 33 38 cgcatATGa
wtGFP-myc 100 7 100 33 100 agaccATGg
S65T-myc 100 80 25 103 agaccATGg
Bex1-myc 100 60 32 62 agaccATGg
Vex1-myc 100 40 25 55 agaccATGg
The results are average of three independent experiments. All values, except for half-lives, are normalized versus wtGFP-myc and expressed as
percentage.
a
GFP mRNA levels.
b
GFP mRNA half-life expressed in hours.
c
GFP protein levels (P).
d
GFP protein half-life expressed in hours.
e
mRNA translation rates.
f
Kozak ribosomal binding sequence [21] ; the ATG start of translation is in capital letters.
FEBS 24684 5-3-01
A. Sacchetti et al./FEBS Letters 492 (2001) 151^155 153
4. Conclusions
In the past, structural and functional analyses of GFP mu-
tants identi¢ed in screening programs have repeatedly raised
questions as to their actual mechanisms of action [4,6,8]. Our
results demonstrate that several commonly engineered altera-
tions in GFP structure have remarkable consequences on
mRNA expression parameters. The major a¡ected mechanism
is the e¤ciency of mRNA translation, that was shown to span
a 200-fold range. Signi¢cant e¡ects were also demonstrated on
protein stability and mRNA transcription rates. These previ-
ously unrecognized e¡ects were found to be critical to the
overall e¤ciency of GFP mutants (Table 1; compare the
translation rates of the di¡erent constructs and the corre-
sponding GFP protein levels). Thus, a strategy of optimiza-
tion of GFP mRNA expression parameters may contribute
considerably to the improvement of GFP expression. The en-
gineering of the 5P and 3P UTs of GFP, in particular, might
permit the reaching of both higher transcription and trans-
lation rates. If GFP mutations are found to have similar con-
sequences on GFP mRNA transcription and translation rates
in lower eukaryotes, high throughput screening strategies for
more e¤cient GFP mutants could be e¤ciently designed in
commonly used yeast strains [47,48].
Acknowledgements: This work was supported by the Italian Associa-
tion for Cancer Research (AIRC), the Italian National Research
Council (Convenzione CNR, Consorzio Mario Negri Sud), and the
Consorzio per la Medicina Tropicale (CMT). A.S. is recipient of a
fellowship from Italian Foundation for Cancer Research (FIRC).
T.E.S. and A.F.N. were recipients of fellowships from the CMT.
References
[1] Prasher, D.C. (1995) Trends Genet. 11, 320^323.
[2] Cubitt, A.B., Heim, R., Adams, S.R., Boyd, A.E., Gross, L.A.
and Tsien, R.Y. (1995) Trends Biochem. Sci. 20, 448^455.
[3] Ludin, B. and Matus, A. (1998) Trends Cell Biol. 8, 72^77.
[4] Tsien, R.Y. (1998) Annu. Rev. Biochem. 67, 509^544.
[5] Sacchetti, A. and Alberti, S. (1999) Nat. Biotechnol. 17, 1046.
[6] Heim, R., Cubitt, A.B. and Tsien, R.Y. (1995) Nature 373, 663^
664.
[7] Anderson, M.T., Tjioe, I.M., Lorincz, M.C., Parks, D.R., Her-
zenberg, L.A. and Nolan, G.P. (1996) Proc. Natl. Acad. Sci.
USA 93, 8508^8511.
[8] Heim, R. and Tsien, R.Y. (1996) Curr. Biol. 6, 178^182.
[9] Crameri, A., Whitehorn, E.A., Tate, E. and Stemmer, W.P.C.
(1996) Nat. Biotechnol. 14, 315^319.
[10] Cormack, B.P., Valdivia, R.H. and Falkow, S. (1996) Gene 173,
33^38.
[11] Siemering, K.R., Golbik, R., Sever, R. and Haselo¡, J. (1996)
Curr. Biol. 6, 1653^1663.
[12] Chal¢e, M., Tu, Y., Euskirchen, G., Ward, W.W. and Prasher,
D.C. (1994) Science 263, 802^805.
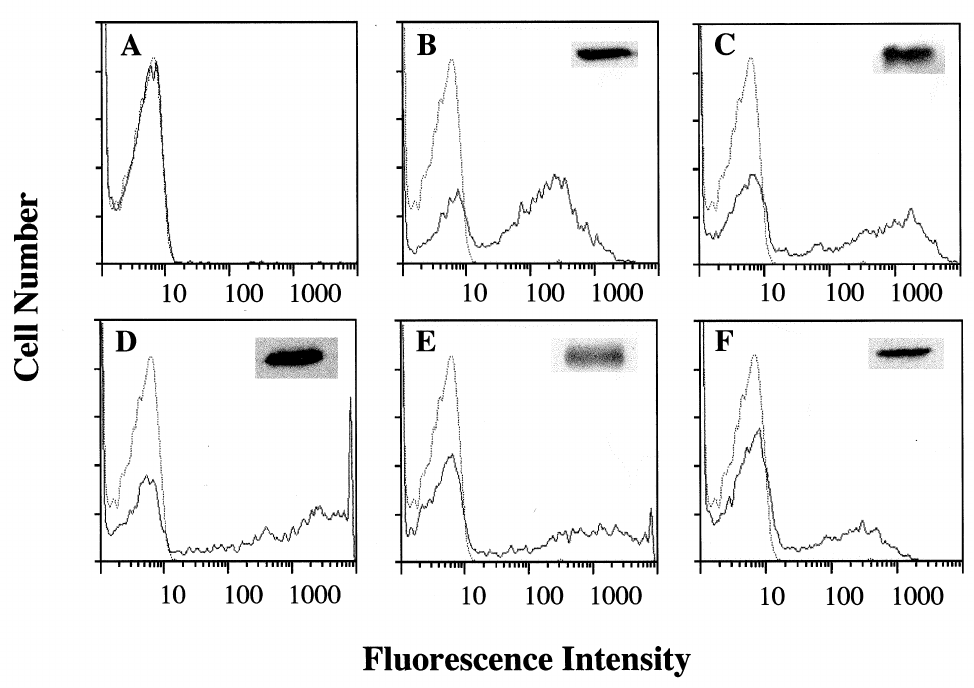
Fig. 3. Expression levels of the transfected GFP constructs. 293T cells were transfected with (A) wtGFP; (B) wtGFP-myc; (C) Bex1-myc; (D)
wtGFP-v5P UT; (E) wtGFP-SB; (F) S65T-myc. Panels show log £uorescence pro¢les of 5000 cells. Solid line: transfected cells. Dotted line:
vector-alone transfected cells. Insets : GFP protein levels, as determined by Western blot analysis. The GFP protein levels in (A) were too low
to be shown on scale with the other samples.
FEBS 24684 5-3-01
A. Sacchetti et al./FEBS Letters 492 (2001) 151^155154
[13] Pantopoulos, K., Johansson, H.E. and Hentze, M.W. (1994)
Prog. Nucleic Acids Res. Mol. Biol. 48, 181^238.
[14] Tanguay, R.L. and Gallie, D.R. (1996) Mol. Cell. Biol. 16, 146^
156.
[15] Al-Qahtani, A. and Mensa-Wilmot, K. (1996) Nucleic Acids Res.
24, 1173^1174.
[16] Munroe, D. and Jacobson, A. (1990) Mol. Cell. Biol. 10, 3441^
3455.
[17] Hann, L.E., Webb, A.C., Cai, J.-M. and Gehrke, L. (1997) Mol.
Cell. Biol. 17, 2005^2013.
[18] Bailey-Serres, J. and Dawe, R.K. (1996) Plant Physiol. 112, 685^
695.
[19] Iida, Y. and Masuda, T. (1996) Nucleic Acids Res. 24, 3313^
3316.
[20] Le, S.Y. and Maizel, J.V.J. (1997) Nucleic Acids Res. 25, 362^
369.
[21] Kozak, M. (1991) J. Biol. Chem. 266, 19867^19870.
[22] Sachs, A.B., Sarnow, P. and Hentze, M.W. (1997) Cell 89, 831^
838.
[23] Afshar-Kharghan, V., Li, C.Q., Khoshnevis-Asl, M. and Lopez,
J.A. (1999) Blood 94, 186^191.
[24] Xiang, S., Parsons, H.K. and Murray, M. (1998) Gene 209, 123^
129.
[25] Bernstein, P.L., Herrick, D.J., Prokipcak, R.D. and Ross, J.
(1992) Genes Dev. 6, 642^654.
[26] Veyrune, J.L., Carillo, S., Vie, A. and Blanchard, J.M. (1995)
Oncogene 11, 2127^2134.
[27] Ross, J. (1995) Microbiol. Rev. 59, 423^450.
[28] Zama, M. (1999) Nucleic Acids Symp. Ser. 42, 81^82.
[29] Xu, Y.H. and Grabowski, G.A. (1999) Mol. Genet. Metab. 68,
441^454.
[30] Waldo, G.S., Standish, B.M., Berendzen, J. and Terwilliger, T.C.
(1999) Nat. Biotechnol. 17, 691^695.
[31] Keiler, K.C., Waller, P.R. and Sauer, R.T. (1996) Science 271,
990^993.
[32] Sacchetti, A., Ciccocioppo, R. and Alberti, S. (2000) Histol. His-
topathol. 15, 101^107.
[33] Sambrook, J., Fritsch, E.F. and Maniatis, T. (1989) Cold Spring
Harbor Laboratory, New York.
[34] Dell'Arciprete, R., Stella, M., Fornaro, M., Ciccocioppo, R.,
Capri, M.G., Naglieri, A.M. and Alberti, S. (1996) J. Histochem.
Cytochem. 44, 629^640.
[35] El-Sewedy, T., Fornaro, M. and Alberti, S. (1998) Int. J. Cancer
75, 324^331.
[36] Matz, M.V., Fradkov, A.F., Labas, Y.A., Savitsky, A.P., Za-
raisky, A.G., Markelov, M.L. and Lukyanov, S.A. (1999) Nat.
Biotechnol. 17, 969^973.
[37] Patterson, G.H., Knobel, S.M., Sharif, W.D., Kain, S.R. and
Piston, D.W. (1997) Biophys. J. 73, 2782^2790.
[38] Ward, W.W., Prentice, H.J., Roth, A.F., Cody, C.W. and
Reeves, S.C. (1982) Photochem. Photobiol. 35, 803^808.
[39] Beyer, D., Skripkin, E., Wadzack, J. and Nierhaus, K.H. (1994)
J. Biol. Chem. 269, 30713^30717.
[40] Dubnau, J. and Struhl, G. (1996) Nature 379, 694^699.
[41] Sacchetti, A., Cappetti, V., Marra, P., Dell'Arciprete, R., El Sew-
edyl, T., Crescenzi, C. and Alberti, S. (2000) J. Cell. Biochem.
[42] Kozak, M. (1986) Cell 44, 283^292.
[43] Kozak, M. (1984) Nucleic Acids Res. 12, 857^872.
[44] Kozak, M. (1989) Mol. Cell. Biol. 9, 5073^5080.
[45] Fedorov, A.N. and Baldwin, T.O. (1997) J. Biol. Chem. 272,
32715^32718.
[46] Harding, H.P., Zhang, Y. and Ron, D. (1999) Nature 397, 271^
274.
[47] Cereghino, G.P. and Cregg, J.M. (1999) Curr. Opin. Biotechnol.
10, 422^427.
[48] Sudbery, P.E. (1996) Curr. Opin. Biotechnol. 7, 517^524.
FEBS 24684 5-3-01
A. Sacchetti et al./FEBS Letters 492 (2001) 151^155 155
