WC-1
Answers to All
Questions and Problems
Chapter 1
1.1 In a few sentences, what were Mendel’s key ideas about
inheritance?
ANS: Mendel postulated transmissible factors—genes—to
explain the inheritance of traits. He discovered that
genes exist in different forms, which we now call alleles.
Each organism carries two copies of each gene. During
reproduction, one of the gene copies is randomly incor-
porated into each gamete. When the male and female
gametes unite at fertilization, the gene copy number is
restored to two. Different alleles may coexist in an organ-
ism. During the production of gametes, they separate
from each other without having been altered by
coexistence.
1.2 Both DNA and RNA are composed of nucleotides. What
molecules combine to form a nucleotide?
ANS: Each nucleotide consists of a sugar, a nitrogen-containing
base, and a phosphate.
1.3 Which bases are present in DNA? Which bases are pres-
ent in RNA? Which sugars are present in each of these
nucleic acids?
ANS: The bases present in DNA are adenine, thymine, gua-
nine, and cytosine; the bases present in RNA are adenine,
uracil, guanine, and cytosine. The sugar in DNA is
deoxyribose; the sugar in RNA is ribose.
1.4 What is a genome?
ANS: A genome is the set of all the DNA molecules that are
characteristic of an organism. Each DNA molecule
forms one chromosome in a cell of the organism.
1.5 The sequence of a strand of DNA is ATTGCCGTC. If
this strand serves as the template for DNA synthesis,
what will be the sequence of the newly synthesized
strand?
ANS: TAACGGCAG
1.6 A gene contains 141 codons. How many nucleotides are
present in the gene’s coding sequence? How many amino
acids are expected to be present in the polypeptide
encoded by this gene?
ANS: There are 3 141 423 nucleotides in the gene’s cod-
ing sequence. Its polypeptide product will contain 141
amino acids.
1.7 The template strand of a gene being transcribed is CTT-
GCCAGT. What will be the sequence of the RNA made
from this template?
ANS: GAACGGUCT
1.8 What is the difference between transcription and
translation?
ANS: Transcription is the production of an RNA chain using a
DNA chain as a template. Translation is the production
of a chain of amino acids—that is, a polypeptide—using
an RNA chain as a template.
1.9 RNA is synthesized using DNA as a template. Is DNA
ever synthesized using RNA as a template? Explain.
ANS: Sometimes, DNA is synthesized from RNA in a process
called reverse transcription. This process plays an impor-
tant role in the life cycles of some viruses.
1.10 The gene for a-globin is present in all vertebrate species.
Over millions of years, the DNA sequence of this gene
has changed in the lineage of each species. Consequently,
the amino acid sequence of a-globin has also changed in
these lineages. Among the 141 amino acid positions in
this polypeptide, human a-globin differs from shark
a-globin in 79 positions; it differs from carp a-globin in
68 and from cow a-globin in 17. Do these data suggest
an evolutionary phylogeny for these vertebrate species?
ANS: The human and cow a-globins are least different; there-
fore, on the assumption that differences in a-globin
re ect the degree of phylogenetic relationship, the
human and the cow are the most closely related organ-
isms among those mentioned. The next closest “relative”
of humans is the carp, and the most distant relative is the
shark.
1.11 Sickle-cell anemia is caused by a mutation in one of the
codons in the gene for b-globin; because of this mutation,
the sixth amino acid in the b-globin polypeptide is a
valine instead of a glutamic acid. A less severe type of ane-
mia is caused by a mutation that changes this same codon
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 1 8/14/2015 6:42:39 PM

2-WC Answers to All Questions and Problems
to one specifying lysine as the sixth amino acid in the
b-globin polypeptide. What word is used to describe the
two mutant forms of this gene? Do you think that an indi-
vidual carrying these two mutant forms of the b-globin
gene would suffer from anemia? Explain.
ANS: The two mutant forms of the b-globin gene are properly
described as alleles. Because neither of the mutant alleles
can specify a “normal” polypeptide, an individual who
carries each of them would probably suffer from
anemia.
1.12 Hemophilia is an inherited disorder in which the blood-
clotting mechanism is defective. Because of this defect,
people with hemophilia may die from cuts or bruises,
especially if internal organs such as the liver, lungs, or
kidneys have been damaged. One method of treatment
involves injecting a blood-clotting factor that has been
puried from blood donations. This factor is a protein
encoded by a human gene. Suggest a way in which mod-
ern genetic technology could be used to produce this
factor on an industrial scale. Is there a way in which the
inborn error of hemophilia could be corrected by human
gene therapy?
ANS: The gene for the human clotting factor could be isolated
from the human genome and transferred into bacteria,
which could then be grown in vats to produce large
amounts of the gene’s protein product. This product could
be isolated from the bacteria, puried, and then injected
into patients to treat hemophilia. Another approach would
be to transfer a normal copy of the clotting factor gene
into the cells of people who have hemophilia. If expressed
properly, the transferred normal gene might be able to
compensate for the mutant allele these people naturally
carry. For this approach to succeed, the normal clotting
factor gene would have to be transferred into the cells that
produce clotting factor, or into their precursors.
Chapter 2
2.1 Carbohydrates and proteins are linear polymers. What
types of molecules combine to form these polymers?
ANS: Sugars combine to form carbohydrates; amino acids
combine to form proteins.
2.2 All cells are surrounded by a membrane; some cells are
surrounded by a wall. What are the differences between
cell membranes and cell walls?
ANS: Cell membranes are made of lipids and proteins; they
have a uid structure. Cell walls are made of more rigid
materials such as cellulose.
2.3 What are the principal differences between prokaryotic
and eukaryotic cells?
ANS: In a eukaryotic cell, the many chromosomes are con-
tained within a membrane-bounded structure called the
nucleus; the chromosomes of prokaryotic cells are not
contained within a special subcellular compartment.
Eukaryotic cells usually possess a well-developed inter-
nal system of membranes and they also have membrane-
bounded subcellular organelles such as mitochondria
and chloroplasts; prokaryotic cells do not typically have
a system of internal membranes (although some do), nor
do they possess membrane-bounded organelles.
2.4 Distinguish between the haploid and diploid states.
What types of cells are haploid? What types of cells are
diploid?
ANS: In the haploid state, each chromosome is represented
once; in the diploid state, each chromosome is repre-
sented twice. Among multicellular eukaryotes, gam-
etes are haploid and somatic cells are diploid.
2.5 Compare the sizes and structures of prokaryotic and
eukaryotic chromosomes.
ANS: Prokaryotic chromosomes are typically (but not always)
smaller than eukaryotic chromosomes; in addition, pro-
karyotic chromosomes are circular, whereas eukaryotic
chromosomes are linear. For example, the circular chro-
mosome of E. coli, a prokaryote, is about 1.4 mm in cir-
cumference. By contrast, a linear human chromosome
may be 10–30 cm long. Prokaryotic chromosomes also
have a comparatively simple composition: DNA, some
RNA, and some protein. Eukaryotic chromosomes are
more complex: DNA, some RNA, and a lot of protein.
2.6 With a focus on the chromosomes, what are the key
events during interphase and M phase in the eukaryotic
cell cycle?
ANS: During interphase, the chromosomes duplicate. During
M phase (mitosis), the duplicated chromosomes, each
consisting of two identical sister chromatids, condense
(a feature of prophase), migrate to the equatorial plane of
the cell (a feature of metaphase), and then split so that
their constituent sister chromatids are separated into dif-
ferent daughter cells (a feature of anaphase); this last
process is called sister chromatid disjunction.
2.7 Which typically lasts longer, interphase or M phase? Can
you explain why one of these phases lasts longer than the
other?
ANS: Interphase typically lasts longer than M phase. During
interphase, DNA must be synthesized to replicate all the
chromosomes. Other materials must also be synthesized
to prepare for the upcoming cell division.
2.8 In what way do the microtubule organizing centers of
plant and animal cells differ?
ANS: The microtubule organizing centers of animal cells have
distinct centrosomes, whereas the microtubule organiz-
ing centers of plant cells do not.
2.9 Match the stages of mitosis with the events they encom-
pass: Stages: (1) anaphase, (2) metaphase, (3) prophase,
and (4) telophase. Events: (a) reformation of the nucleo-
lus, (b) disappearance of the nuclear membrane,
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 2 8/14/2015 6:42:39 PM

Answers to All Questions and Problems WC-3
(c) condensation of the chromosomes, (d) formation of
the mitotic spindle, (e) movement of chromosomes to
the equatorial plane, (f) movement of chromosomes to
the poles, (g) decondensation of the chromosomes, (h)
splitting of the centromere, and (i) attachment of micro-
tubules to the kinetochore.
ANS: (1) Anaphase: (f), (h); (2) metaphase: (e), (i); (3) prophase:
(b), (c), (d); (4) telophase: (a), (g).
2.10 Arrange the following events in the correct temporal
sequence during eukaryotic cell division, starting with
the earliest: (a) condensation of the chromosomes,
(b) movement of chromosomes to the poles, (c) duplica-
tion of the chromosomes, (d) formation of the nuclear
membrane, (e) attachment of microtubules to the kineto-
chores, and (f) migration of centrosomes to positions on
opposite sides of the nucleus.
ANS: (c), (f), (a), (e), (b), (d).
2.11 In human beings, the gene for b-globin is located on chro-
mosome 11, and the gene for a-globin, which is another
component of the hemoglobin protein, is located on chro-
mosome 16. Would these two chromosomes be expected to
pair with each other during meiosis? Explain your answer.
ANS: Chromosomes 11 and 16 would not be expected to pair
with each other during meiosis; these chromosomes are
heterologues, not homologues.
2.12 A sperm cell from the fruit y Drosophila melanogaster
contains four chromosomes. How many chromosomes
would be present in a spermatogonial cell about to enter
meiosis? How many chromatids would be present in a
spermatogonial cell at metaphase I of meiosis? How
many would be present at metaphase II?
ANS: There are eight chromosomes in a Drosophila spermato-
gonial cell about to enter meiosis. There are 16 chroma-
tids in a Drosophilia spermatogonial cell at metaphase I of
meiosis. There are eight chromatids in a Drosophilia cell
at metaphase II of meiosis.
2.13 Does crossing over occur before or after chromosome
duplication in cells going through meiosis?
ANS: Crossing over occurs after chromosomes have duplicated
in cells going through meiosis.
2.14 What visible characteristics of chromosomes indicate
that they have undergone crossing over during meiosis?
ANS: The chiasmata, which are visible late in prophase I of
meiosis, indicate that chromosomes have crossed over.
2.15 During meiosis, when does chromosome disjunction
occur? When does chromatid disjunction occur?
ANS: Chromosome disjunction occurs during anaphase I.
Chromatid disjunction occurs during anaphase II.
2.16 In Arabidopsis, is leaf tissue haploid or diploid? How
many nuclei are present in the female gametophyte?
How many are present in the male gametophyte? Are
these nuclei haploid or diploid?
ANS: Leaf tissue is diploid. The female gametophyte contains
eight identical haploid nuclei. The male gametophyte
contains three identical haploid nuclei.
2.17 From the information given in Table 2.1 in this chapter,
is there a relationship between genome size (measured in
base pairs of DNA) and gene number? Explain.
ANS: Among eukaryotes, there does not seem to be a clear
relationship between genome size and gene number. For
example, humans, with 3.2 billion base pairs of genomic
DNA, have about 20,500 genes, and Arabidopsis plants,
with about 150 million base pairs of genomic DNA, have
roughly the same number of genes as humans. However,
among prokaryotes, gene number is rather tightly cor-
related with genome size, probably because there is so
little nongenic DNA.
2.18 Are the synergid cells in an Arabidopsis female gameto-
phyte genetically identical to the egg cell nestled between
them?
ANS: Yes.
2.19 A cell of the bacterium Escherichia coli, a prokaryote, con-
tains one chromosome with about 4.6 million base pairs
of DNA comprising 4288 protein-encoding genes. A cell
of the yeast Saccharomyces cerevisiae, a eukaryote, contains
about 12 million base pairs of DNA comprising 6268
genes, and this DNA is distributed over 16 distinct chro-
mosomes. Are you surprised that the chromosome of a
prokaryote is larger than some of the chromosomes of a
eukaryote? Explain your answer.
ANS: It is a bit surprising that yeast chromosomes are, on aver-
age, smaller than E. coli chromosomes because, as a rule,
eukaryotic chromosomes are larger than prokaryotic
chromosomes. Yeast is an exception because its genome—
not quite three times the size of the E. coli genome—is
distributed over 16 separate chromosomes.
2.20 Given the way that chromosomes behave during meiosis,
is there any advantage for an organism to have an even
number of chromosome pairs (such as Drosophila does),
as opposed to an odd number of chromosome pairs (such
as human beings do)?
ANS: No, there is no advantage associated with an even num-
ber of chromosomes. As long as the chromosomes come
in pairs, they will be able to synapse during prophase I
and then disjoin during anaphase I to distribute the
genetic material properly to the two daughter cells.
2.21 In owering plants, two nuclei from the pollen grain par-
ticipate in the events of fertilization. With which nuclei
from the female gametophyte do these nuclei combine?
What tissues are formed from the fertilization events?
ANS: One of the pollen nuclei fuses with the egg nucleus in the
female gametophyte to form the zygote, which then
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 3 8/14/2015 6:42:39 PM

4-WC Answers to All Questions and Problems
develops into an embryo and ultimately into a sporo-
phyte. The other genetically functional pollen nucleus
fuses with two nuclei in the female gametophyte to form
a triploid nucleus, which then develops into a triploid
tissue, the endosperm; this tissue nourishes the develop-
ing plant embryo.
2.22 The mouse haploid genome contains about 2.9 10
9
nucleotide pairs of DNA. How many nucleotide pairs of
DNA are present in each of the following mouse cells:
(a) somatic cell, (b) sperm cell, (c) fertilized egg, (d) pri-
mary oocyte, (e) rst polar body, and (f) secondary
spermatocyte?
ANS: (a) 5.8 10
9
nucleotide pairs (np); (b) 2.9 10
9
np;
(c) 5.8 10
9
np; (d) 11.6 10
9
np; (e) 5.8 10
9
np;
and (f) 5.8 10
9
np
2.23 Arabidopsis plants have 10 chromosomes (ve pairs) in
their somatic cells. How many chromosomes are present
in each of the following: (a) egg cell nucleus in the female
gametophyte, (b) generative cell nucleus in a pollen
grain, (c) fertilized endosperm nucleus, and (d) fertilized
egg nucleus?
ANS: (a) 5, (b) 5, (c) 15, (d) 10.
Chapter 3
3.1 On the basis of Mendel’s observations, predict the results
from the following crosses with peas: (a) a tall (dominant
and homozygous) variety crossed with a dwarf variety;
(b) the progeny of (a) self-fertilized; (c) the progeny from
(a) crossed with the original tall parent; (d) the progeny
of (a) crossed with the original dwarf parent.
ANS: (a) All tall; (b) 3/4 tall, 1/4 dwarf; (c) all tall; (d) 1/2 tall,
1/2 dwarf.
3.2 Mendel crossed pea plants that produced round seeds
with those that produced wrinkled seeds and self-fertil-
ized the progeny. In the F
2
, he observed 5474 round
seeds and 1850 wrinkled seeds. Using the letters W and
w for the seed texture alleles, diagram Mendel’s crosses,
showing the genotypes of the plants in each generation.
Are the results consistent with the Principle of
Segregation?
ANS: Round (WW ) wrinkled (ww) → F
1
round (Ww); F
1
self-fertilized → F
2
3/4 round (2 WW; 1 Ww), 1/4 wrin-
kled (ww). The expected results in the F
2
are 5493 round,
1831 wrinkled. To compare the observed and expected
results, compute c
2
with one degree of freedom;
(5474 5493)
2
/5493 (1850 1831)
2
/1831 0.263,
which is not signicant at the 5% level. Thus, the results
are consistent with the Principle of Segregation.
3.3 A geneticist crossed wild, gray-colored mice with white
(albino) mice. All the progeny were gray. These progeny
were intercrossed to produce an F
2
, which consisted of
198 gray and 72 white mice. Propose a hypothesis to
explain these results, diagram the crosses, and compare
the results with the predictions of the hypothesis.
ANS: The data suggest that coat color is controlled by a single
gene with two alleles, C (gray) and c (albino), and that C
is dominant over c. On this hypothesis, the crosses are
gray (CC) albino (cc) → F
1
gray (Cc); F
1
F
1
→ 3/4
gray (2 CC: 1 Cc), 1/4 albino (cc). The expected results in
the F
2
are 203 gray and 67 albino. To compare the
observed and expected results, compute c
2
with one
degree of freedom: (198 203)
2
/203 (67 72)
2
/72
0.470, which is not signicant at the 5% level. Thus, the
results are consistent with the hypothesis.
3.4 A woman has a rare abnormality of the eyelids called
ptosis, which prevents her from opening her eyes com-
pletely. This condition is caused by a dominant allele,
P. The woman’s father had ptosis, but her mother had
normal eyelids. Her father’s mother had normal
eyelids.
(a) What are the genotypes of the woman, her father, and
her mother?
(b) What proportion of the woman’s children will have
ptosis if she marries a man with normal eyelids?
ANS: (a) Woman’s genotype Pp, father’s genotype Pp, mother’s
genotype pp; (b) ½
3.5 In pigeons, a dominant allele C causes a checkered pat-
tern in the feathers; its recessive allele c produces a plain
pattern. Feather coloration is controlled by an indepen-
dently assorting gene; the dominant allele B produces
red feathers, and the recessive allele b produces brown
feathers. Birds from a true-breeding checkered, red vari-
ety are crossed to birds from a true-breeding plain,
brown variety.
(a) Predict the phenotype of their progeny.
(b) If these progeny are intercrossed, what phenotypes
will appear in the F
2
and in what proportions?
ANS: (a) Checkered, red (CC BB) plain, brown (cc bb) → F
1
all checkered, red (Cc Bb); (b) F
2
progeny: 9/16 check-
ered, red (C- B-), 3/16 plain, red (cc B-), 3/16 checkered,
brown (C- bb), 1/16 plain, brown (cc bb).
3.6 In mice, the allele C for colored fur is dominant over the
allele c for white fur, and the allele V for normal behavior
is dominant over the allele v for waltzing behavior, a
form of dis-coordination. Given the genotypes of the
parents in each of the following crosses:
(a) Colored, normal mice mated with white, normal mice
produced 29 colored, normal, and 10 colored, waltzing
progeny
(b) Colored, normal mice mated with colored, normal
mice produced 38 colored, normal, 15 colored, waltzing,
11 white, normal, and 4 white, waltzing progeny
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 4 8/14/2015 6:42:39 PM
Answers to All Questions and Problems WC-5
(c) Colored, normal mice mated with white, waltzing
mice produced 8 colored, normal, 7 colored, waltzing,
9 white, normal, and 6 white, waltzing progeny.
ANS: (a) colored, normal (CC Vv) white, normal (cc Vv)
(b) colored, normal (Cc Vv) colored, normal (Cc Vv);
(c) colored, normal (Cc Vv) white, waltzing (cc vv).
3.7 In rabbits, the dominant allele B causes black fur and the
recessive allele b causes brown fur; for an independently
assorting gene, the dominant allele R causes long fur and
the recessive allele r (for rex) causes short fur. A homozy-
gous rabbit with long, black fur is crossed with a rabbit
with short, brown fur, and the offspring are intercrossed.
In the F
2
, what proportion of the rabbits with long, black
fur will be homozygous for both genes?
ANS: Among the F
2
progeny with long, black fur, the geno-
typic ratio is 1 BB RR: 2 BB Rr: 2 Bb RR: 4 Bb Rr; thus, 1/9
of the rabbits with long, black fur are homozygous for
both genes.
3.8 In shorthorn cattle, the genotype RR causes a red coat,
the genotype rr causes a white coat, and the genotype
Rr causes a roan coat. A breeder has red, white, and
roan cows and bulls. What phenotypes might be
expected from the following matings and in what
proportions?
(a) Red red
(b) Red roan
(c) Red white
(d) Roan roan.
ANS: (a) All red; (b) 1/2 red, 1/2 roan; (c) all roan; (d) 1/4 red,
1/2 roan, 1/4 white
3.9 How many different kinds of F
1
gametes, F
2
genotypes,
and F
2
phenotypes would be expected from the following
crosses:
(a) AA aa;
(b) AA BB aa bb;
(c) AA BB CC aa bb cc?
(d) What general formulas are suggested by these
answers?
ANS:
F
1
Gametes F
2
Genotypes F
2
Phenotypes
(a) 2 3 2
(b) 2 2 4 3 3 9 2 2 4
(c) 2 2 2 8 3 3 3 27 2 2 2 8
(d) 2
n
3
n
2
n
, where n is the
number of genes
3.10 A researcher studied six independently assorting genes in
a plant. Each gene has a dominant and a recessive allele:
R black stem, r red stem; D tall plant, d dwarf plant; C full
pods, c constricted pods; O round fruit, o oval fruit; H
hairless leaves, h hairy leaves; W purple ower, w white
ower. From the cross (P1) Rr Dd cc Oo Hh Ww (P2)
Rr dd Cc oo Hh ww,
(a) How many kinds of gametes can be formed by P1?
(b) How many genotypes are possible among the prog-
eny of this cross?
(c) How many phenotypes are possible among the
progeny?
(d) What is the probability of obtaining the Rr Dd cc Oo
hh ww genotype in the progeny?
(e) What is the probability of obtaining a black, dwarf,
constricted, oval, hairy, purple phenotype in the
progeny?
ANS: (a) 2 2 1 2 2 2 32; (b) 3 2 2 2 3
2 144; (c) 2 2 2 2 2 2 64; (d) (1/2)
(1/2) (1/2) (1/2) (1/4) (1/2) 1/128; (e) (3/4)
(1/2) (1/2) (1/2) (1/4) (1/2) 3/256.
3.11 For each of the following situations, determine the
degrees of freedom associated with the c
2
statistic and
decide whether or not the observed c
2
value warrants
acceptance or rejection of the hypothesized genetic ratio.
Hypothesized Ratio Observed−c
2
(a) 3:1 7.0
(b) 1:2:1 7.0
(c) 1:1:1:1 7.0
(d) 9:3:3:1 5.0
ANS: (a) 1, reject; (b) 2, reject; (c) 3, accept; (d) 3, accept.
3.12 Mendel testcrossed pea plants grown from yellow, round
F
1
seeds to plants grown from green, wrinkled seeds and
obtained the following results: 31 yellow, round; 26
green, round; 27 yellow, wrinkled; and 26 green, wrin-
kled. Are these results consistent with the hypothesis that
seed color and seed texture are controlled by indepen-
dently assorting genes, each segregating two alleles?
ANS: On the hypothesis, the expected number in each class
is 27.5; c
2
with three degrees of freedom is calculated as
(31 27.5)
2
/27.5 (26 27.5)
2
/27.5 (27 27.5)
2
/27.5
(26 27.5)
2
/27.5 0.618, which is not signicant at the
5% level. Thus, the results are consistent with the hypoth-
esis of two independently assorting genes, each segregating
two alleles.
3.13 Perform a chi-square test to determine if an observed
ratio of 30 tall to 20 dwarf pea plants is consistent with
an expected ratio of 1:1 from the cross Dd dd.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 5 8/14/2015 6:42:39 PM
6-WC Answers to All Questions and Problems
ANS: c
2
(30 25)
2
/25 (20 25)
2
/25 2, which is less
than 3.84, the 5 percent critical value for a chi-square
statistic with one degree of freedom; consequently, the
observed segregation ratio is consistent with the expected
ratio of 1:1.
3.14 Seed capsules of the Shepherd’s purse are either triangu-
lar or ovoid. A cross between a plant with triangular seed
capsules and a plant with ovoid seed capsules yielded F
1
hybrids that all had triangular seed capsules. When these
F
1
hybrids were intercrossed, they produced 80 F
2
plants,
72 of which had triangular seed capsules and 8 of which
had ovoid seed capsules. Are these results consistent with
the hypothesis that capsule shape is determined by a sin-
gle gene with two alleles?
ANS: If capsule shape is determined by a single gene with two
alleles, the F
2
plants should segregate in a 3:1 ratio. To
test for agreement between the observed segregation
data and the expected ratio, compute the expected num-
ber of plants with either triangular or ovoid seed cap-
sules: (3/4) 80 60 triangular and (1/4) 80 20
ovoid; then compute a c
2
statistic with one degree of
freedom: c
2
(72 60)
2
/60 (8 20)
2
/20 9.6, which
exceeds the critical value of 3.84. Consequently, the data
are inconsistent with the hypothesis that capsule shape is
determined by a single gene with two alleles.
3.15 Albinism in humans is caused by a recessive allele a.
From marriages between people known to be carriers
(Aa) and people with albinism (aa), what proportion of
the children would be expected to have albinism? Among
three children, what is the chance of one without albi-
nism and two with albinism?
ANS: Half the children from Aa aa matings would have
albinism. In a family of three children, the chance that
one will be unaffected and two affected is 3 (1/2)
1
(1/2)
2
3/8.
3.16 If both husband and wife are known to be carriers of the
allele for albinism, what is the chance of the following
combinations in a family of four children: (a) all four
unaffected; (b) three unaffected and one affected; (c) two
unaffected and two affected; (d) one unaffected and three
affected?
ANS: (a) (3/4)
4
81/256; (b) 4 (3/4)
3
(1/4)
1
108/256; (c)
6 (3/4)
2
(1/4)
2
54/256; (d) 4 (3/4)
1
(1/4)
3
12/256.
3.17 In humans, cataracts in the eyes and fragility of the bones
are caused by dominant alleles that assort independently.
A man with cataracts and normal bones marries a woman
without cataracts but with fragile bones. The man’s
father had normal eyes, and the woman’s father had nor-
mal bones. What is the probability that the rst child of
this couple will (a) be free from both abnormalities; (b)
have cataracts but not have fragile bones; (c) have fragile
bones but not have cataracts; (d) have both cataracts and
fragile bones?
ANS: Man (Cc ff ) woman (cc Ff ). (a) cc ff, (1/2) (1/2) 1/4;
(b) Cc ff, (1/2) (1/2) 1/4; (c) cc Ff, (1/2) (1/2) 1/4;
(d) Cc Ff, (1/2) (1/2) 1/4.
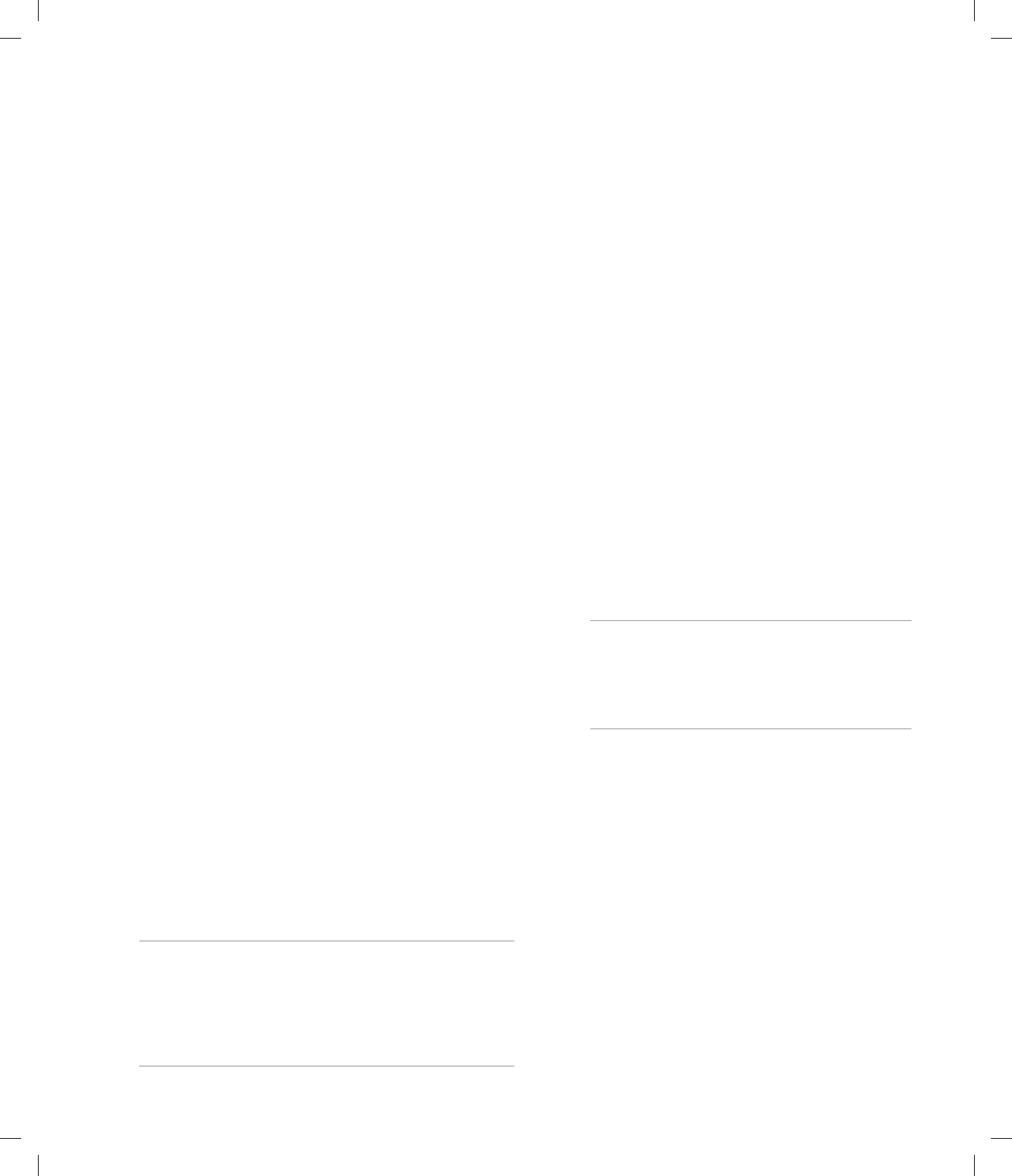
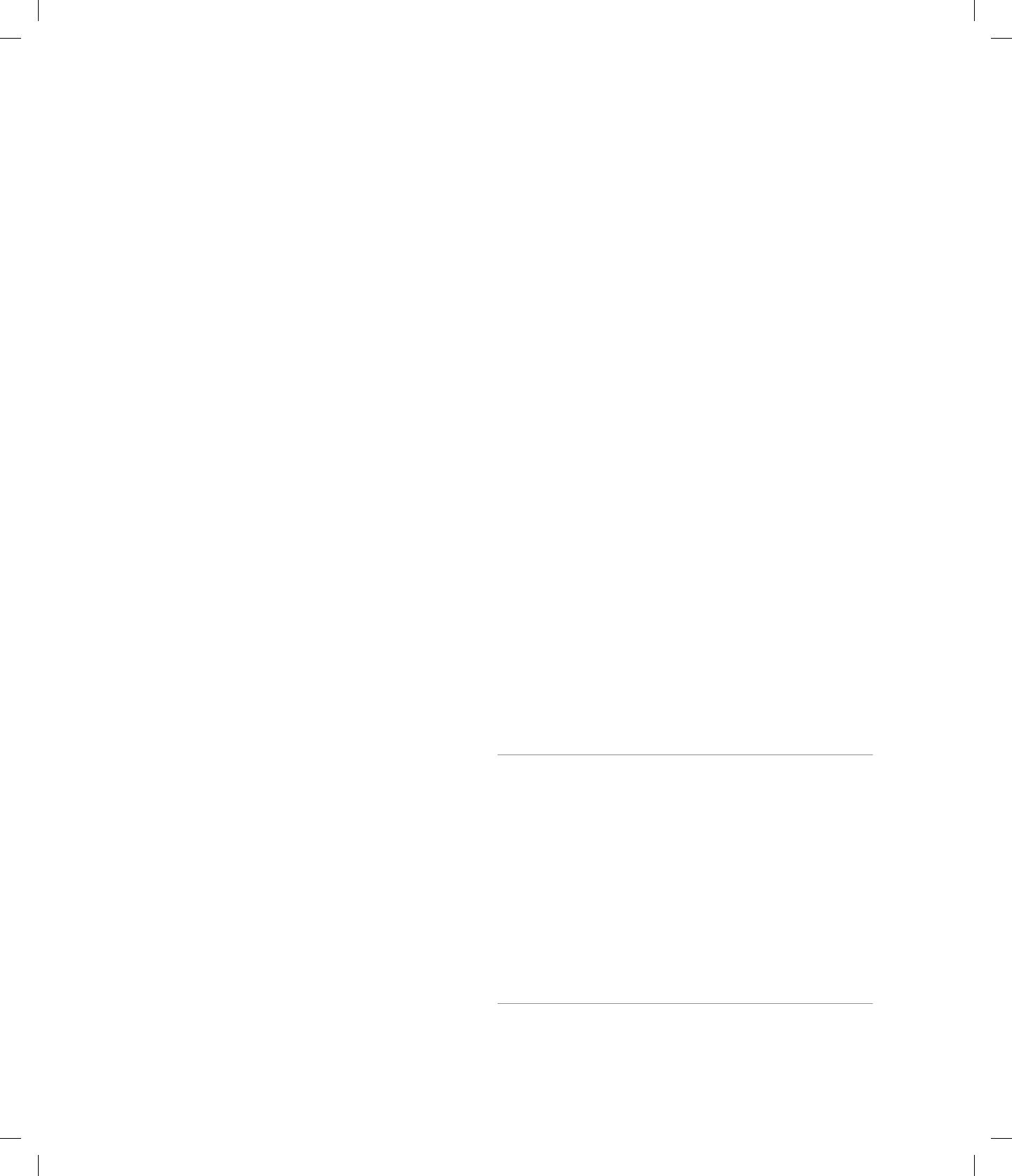
3.18 In generation V in the pedigree in Figure 3.15, what is
the probability of observing seven children without the
cancer-causing mutation and two children with this
mutation among a total of nine children?
ANS: 9!/(7! 2!) (1/2)
7
(1/2)
2
0.07
3.19 If a man and a woman are heterozygous for a gene, and if
they have three children, what is the chance that all three
will also be heterozygous?
ANS: (1/2)
3
1/8
3.20 If four babies are born on a given day: (a) What is the
chance that two will be boys and two will be girls?
(b) What is the chance that all four will be girls? (c) What
combination of boys and girls among four babies is most
likely? (d) What is the chance that at least one baby will
be a girl?
ANS: (a) 4 (1/2)
2
(1/2)
2
4/16; (b) (1/2)
4
1/16; (c) 2
boys, girls; (d) 1 probability that all four are boys 1
(1/2)
4
15/16.
3.21 In a family of six children, what is the chance that at least
three are girls?
ANS: (20/64) (15/64) (6/64) (1/64) 42/64
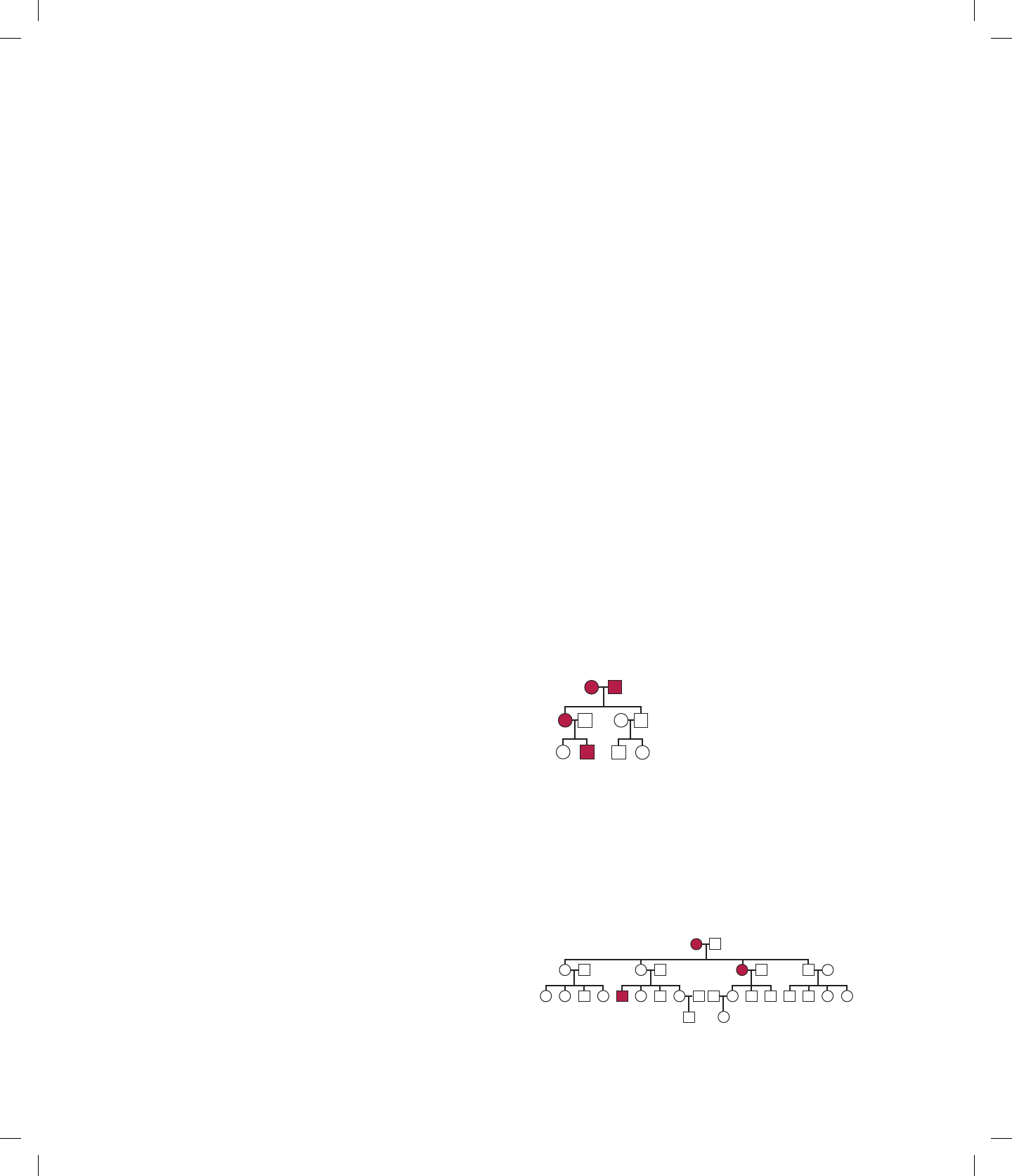
3.22 The following pedigree shows the inheritance of a domi-
nant trait. What is the chance that the offspring of the
following matings will show the trait: (a) III-1 III-3;
(b) III-2 III-4?
I
II
III
21
34
21
3421
ANS: (a) zero; (b) 1/2
3.23 The following pedigree shows the inheritance of a reces-
sive trait. Unless there is evidence to the contrary, assume
that the individuals who have married into the family do
not carry the recessive allele. What is the chance that the
offspring of the following matings will show the trait: (a)
III-1 III-12; (b) II-4 III-14; (c) III-6 III-13; (d)
IV-1 IV-2?
I
12
1
1234
2
II
III
3
5678
4 7
14 15 16 17
85
109
21
11 12 13
6
ANS: (a) (1/2) (1/4) 1/8; (b) (1/2) (1/2) (1/4) 1/16;
(c) (2/3) (1/4) 1/6; (d) (2/3) (1/2) (1/2) (1/4)
1/24
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 6 8/14/2015 6:42:40 PM
Answers to All Questions and Problems WC-7
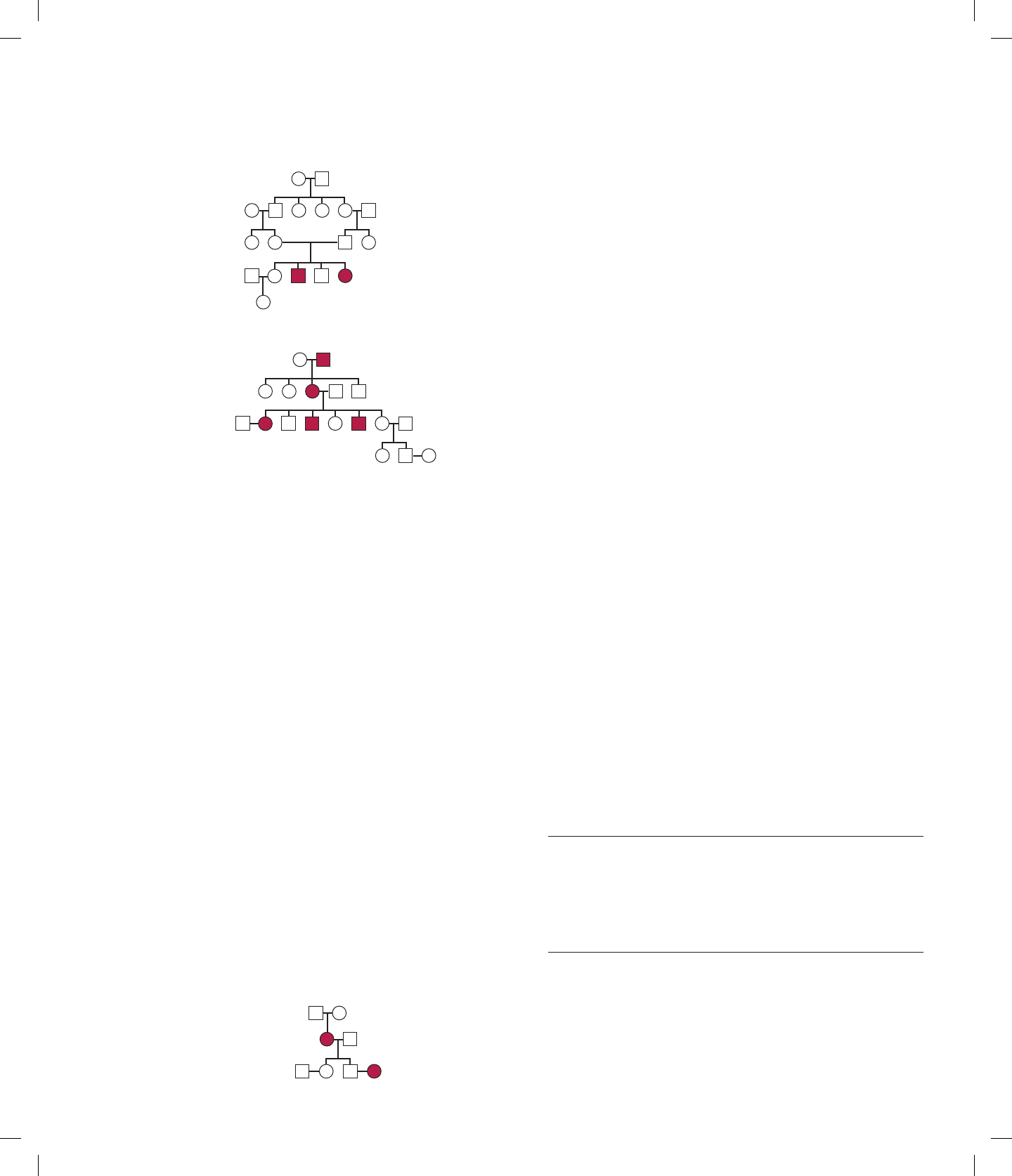
3.24 In the following pedigrees, determine whether the trait is
more likely to be due to a dominant or a recessive allele.
Assume the trait is rare in the population.
I
12
1
12
23
II
III
IV
V
(a)
5
34
6
2345
1
1
I
II
III
IV
12
1 234
(b)
5
45123678
123
ANS: (a) Recessive; (b) dominant.
3.25 In pedigree (b) of Problem 3.24, what is the chance that
the couple III-1 and III-2 will have an affected child?
What is the chance that the couple IV-2 and IV-3 will
have an affected child?
ANS: For III-1 III-2, the chance of an affected child is 1/2.
For IV-2 IV-3, the chance is zero.
3.26 Peas heterozygous for three independently assorting
genes were intercrossed.
(a) What proportion of the offspring will be homozy-
gous for all three recessive alleles?
(b) What proportion of the offspring will be homozy-
gous for all three genes?
(c) What proportion of the offspring will be homozy-
gous for one gene and heterozygous for the other two?
(d) What proportion of the offspring will be homozy-
gous for the recessive allele of at least one gene?
ANS: (a) (1/4)
3
1/64; (b) (1/2)
3
1/8; (c) 3 (1/2)
1
(1/2)
2
3/8; (d) 1 probability that the offspring is not
homozygous for the recessive allele of any gene 1 (3/4)
3
37/64.
3.27 The following pedigree shows the inheritance of a reces-
sive trait. What is the chance that the couple III-3 and
III-4 will have an affected child?
I
12
12
II
III
2413
ANS: 1/2
3.28 A geneticist crosses tall pea plants with short pea plants.
All the F
1
plants are tall. The F
1
plants are then allowed
to self-fertilize, and the F
2
plants are classied by height:
62 tall and 26 short. From these results, the geneticist
concludes that shortness in peas is due to a recessive
allele (s) and that tallness is due to a dominant allele (S).
On this hypothesis, 2/3 of the tall F
2
plants should be
heterozygous Ss. To test this prediction, the geneticist
uses pollen from each of the 62 tall plants to fertilize the
ovules of emasculated owers on short pea plants. The
next year, three seeds from each of the 62 crosses are
sown in the garden and the resulting plants are grown to
maturity. If none of the three plants from a cross is short,
the male parent is classied as having been homozygous
SS; if at least one of the three plants from a cross is short,
the male parent is classied as having been heterozygous
Ss. Using this system of progeny testing, the geneticist
concludes that 29 of the 62 tall F
2
plants were homozy-
gous SS and that 33 of these plants were heterozygous Ss.
(a) Using the chi-square procedure, evaluate these results
for goodness of t to the prediction that 2/3 of the tall F
2
plants should be heterozygous.
(b) Informed by what you read in A Milestone in Genet-
ics: Mendel’s 1866 Paper, which you can nd in the Stu-
dent Companion Site, explain why the geneticist’s
procedure for classifying tall F
2
plants by genotype is not
denitive.
(c) Adjust for the uncertainty in the geneticist’s classi-
cation procedure and calculate the expected frequencies
of homozygotes and heterozygotes among the tall
F
2
plants.
(d) Evaluate the predictions obtained in (c) using the chi-
square procedure.
ANS: (a) The observed numbers, expected numbers, and chi-
square calculation are laid out in the following table:
Observed Expected (Obs − Exp)
2
/Exp
Dominant
homozygotes
(SS)
29
62 1/3 20.7
3.33
Heterozygotes
(Ss)
33
62 2/3 41.3
1.67
Total 62 62 5.00
The total chi-square value is greater than the critical
value for a chi-square statistic with one degree of free-
dom (3.84). Therefore, we reject the hypothesis that the
expected proportions are 1/3 and 2/3.
(b) The problem with the geneticist’s classication
procedure is that it allows for a heterozygote to be
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 7 8/14/2015 6:42:41 PM
8-WC Answers to All Questions and Problems
misclassied as a homozygote if none of its three prog-
eny shows the recessive (short) phenotype. The proba-
bility of this event is 1/2 for any one offspring—therefore
(1/2)
3
1/8 for all three offspring.
(c) The predicted frequencies must take into account the
probability of misclassifying a heterozygote as a homo-
zygote. The frequency of heterozygotes expected a priori
(2/3) must be decreased by the probability of misclassi-
cation (1/8); thus, the predicted frequency of heterozy-
gotes is 62 (2/3) (1 1/8) 62 (7/12) 36.2.
The predicted frequency of homozygotes is obtained by
subtraction: 62 – 36.2 25.8.
(d) The chi-square calculation is (29 25.8)
2
/25.8 (33
36.2)
2
/36.2 0.68, which is much less than the critical
value for a chi-square statistic with one degree of free-
dom. Therefore, we tentatively accept the idea that
adjusting for the probability of misclassication explains
the observed data.
3.29 A researcher who has been studying albinism has identi-
ed a large group of families with four children in which
at least one child shows albinism. None of the parents in
this group of families shows albinism. Among the chil-
dren, the ratio of those without albinism to those with
albinism is 1.7:1. The researcher is surprised by this
result because he thought that a 3:1 ratio would be
expected on the basis of Mendel’s Principle of Segrega-
tion. Can you explain the apparently non-Mendelian
segregation ratio in the researcher’s data?
ANS: The researcher has obtained what appears to be a non-
Mendelian ratio because he has been studying only fami-
lies in which at least one child shows albinism. In these
families, both parents are heterozygous for the mutant
allele that causes albinism. However, other couples in the
population might also be heterozygous for this allele but,
simply due to chance, have failed to produce a child with
albinism. If a man and a woman are both heterozygous
carriers of the mutant allele, the chance that a child they
produce will not have albinism is 3/4. The chance that
four children they produce will not have albinism is
therefore (3/4)
4
0.316. In the entire population of fami-
lies in which two heterozygous parents have produced a
total of four children, the average number of affected
children is 1. Among families in which two heterozygous
parents have produced at least one affected child among a
total of four children, the average must be greater than 1.
To calculate this conditional average, let us denote the
number of children with albinism by x, and the probabil-
ity that exactly x of the four children have albinism by
P(x). The average number of affected children among
families in which at least one of the four children is
affected—that is, the conditional average—is therefore
SxP(x)/(1 P(0)), where the sum starts at x 1 and ends
at x 4. We start the sum at x 1 because we must
exclude those cases in which none of the four children is
affected. The divisor (1 P(0)) is the probability that the
couple has had at least one affected child among their
four children. Now P(0) 0.316 and SxP(x) 1. There-
fore, the average we seek is simply 1/(1 0.316) 1.46.
If, in the subset of families with at least one affected child,
the average number of affected children is 1.46, then the
average number of unaffected children is 4 – 1.46 2.54.
Thus, the expected ratio of unaffected to affected chil-
dren in these families is 2.54:1.46, or 1.74:1, which is
what the researcher has observed.
Chapter 4
4.1 What blood types could be observed in children born to
a woman who has blood type M and a man who has
blood type MN?
ANS: M and MN.
4.2 In rabbits, coloration of the fur depends on alleles of the
gene c. From information given in the chapter, what phe-
notypes and proportions would be expected from the fol-
lowing crosses: (a) c
c
cc; (b) c
c c
c; (c) c
c
h
c
c
ch
;
(d) cc
ch
cc; (e) c
c
h
c
c; (f) c
h
c cc?
ANS: (a) All wild-type; (b) 3/4 wild-type, 1/4 albino; (c) 3/4
wild-type, 1/4 chinchilla; (d) 1/2 chinchilla, 1/2 albino;
(e) 3/4 wild-type, 1/4 Himalayan; (f) 1/2 Himalayan, 1/2
albino.
4.3 In mice, a series of ve alleles determines fur color. In
order of dominance, these alleles are as follows: A
Y
, yel-
low fur but homozygous lethal; A
L
, agouti with light
belly; A
, agouti (wild-type); a
t
, black and tan; and a,
black. For each of the following crosses, give the coat
color of the parents and the phenotypic ratios expected
among the progeny: (a) A
Y
A
L
A
Y
A
L
; (b) A
Y
a A
L
a
t
;
(c) a
t
a A
Y
a; (d) A
L
a
t
A
L
A
L
; (e) A
L
A
L
A
Y
A
; (f) A
a
t
a
t
a; (g) a
t
a aa; (h) A
Y
A
L
A
a
t
; and (i) A
Y
a
L
A
Y
A
.
ANS:
Parents Offspring
(a) Yellow yellow 2 yellow: 1 light belly
(b) Yellow light belly 2 yellow: 1 light belly:
1 black and tan
(c) Black and tan yellow 2 yellow: 1 black and tan:
1 black
(d) Light belly light belly All light belly
(e) Light belly yellow 1 yellow: 1 light belly
(f) Agouti black and tan 1 agouti: 1 black and tan
(g) Black and tan black 1 black and tan: 1 black
(h) Yellow agouti 1 yellow: 1 light belly
(i) Yellow yellow 2 yellow: 1 light belly
4.4 In several plants, such as tobacco, primrose, and red clo-
ver, combinations of alleles in eggs and pollen have been
found to inuence the reproductive compatibility of the
plants. Homozygous combinations, such as S
1
S
1
, do not
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 8 8/14/2015 6:42:41 PM

Answers to All Questions and Problems WC-9
develop because S
1
pollen is not effective on S
1
stigmas.
However, S
1
pollen is effective on S
2
S
3
stigmas. What
progeny might be expected from the following crosses
(seed parent written rst): (a) S
1
S
2
S
2
S
3
;
(b) S
1
S
2
S
3
S
4
;
(c) S
4
S
5
S
4
S
5
; and (d) S
3
S
4
S
5
S
6
? .
ANS: (a) S
1
S
2
, S
1
S
3
, S
2
S
3
; (b) S
1
S
3
, S
1
S
4
, S
2
S
3
, S
2
S
4
; (c) S
4
S
5
;
(d) S
3
S
5
, S
3
S
6
, S
4
S
5
, S
4
S
6
.
4.5 From information in the chapter about the ABO blood
types, what phenotypes and ratios are expected from the
following matings: (a) I
A
I
A
I
B
I
B
; (b) I
A
I
B
ii; (c) I
A
i
I
B
i; and (d) I
A
i ii;
ANS: (a) All AB; (b) 1 A: 1 B; (c) 1 A: 1 B: 1 AB: 1 O; (d) 1 A: 1 O.
4.6 A woman with type O blood gave birth to a baby, also
with type O blood. The woman stated that a man with
type AB blood was the father of the baby. Is there any
merit to her statement?
ANS: No. The woman must be ii; if her mate is I
A
I
B
; they could
not have an ii child.
4.7 A woman with type AB blood gave birth to a baby with
type B blood. Two different men claim to be the father.
One has type A blood, the other has type B blood. Can
the genetic evidence decide in favor of either?
ANS: No. The woman is I
A
I
B
. One man could be either I
A
I
A
or
I
A
i; the other could be either I
B
I
B
or I
B
i. Given the uncer-
tainty in the genotype of each man, either could be the
father of the child.
4.8 The ower colors of plants in a particular population
may be blue, purple, turquoise, light blue, or white. A
series of crosses between different members of the popu-
lation produced the following results:
Cross Parents Progeny
1
Purple
blue
All purple
2
Purple
purple
76 purple, 25 turquoise
3
Blue
blue
86 blue, 29 turquoise
4
Purple
turquoise
49 purple, 52 turquoise
5
Purple
purple
69 purple, 22 blue
6
Purple
blue
50 purple, 51 blue
7
Purple
blue
54 purple, 26 blue,
25 turquoise
8
Turquoise
turquoise
All turquoise
9
Purple
blue
49 purple, 25 blue,
23 light blue
10
Light blue
light blue
60 light blue,
29 turquoise, 31 white
11
Turquoise
white
All light blue
12
White
white
All white
13
Purple
white
All purple
How many genes and alleles are involved in the inheri-
tance of ower color? Indicate all possible genotypes for
the following phenotypes: (a) purple; (b) blue; (c) tur-
quoise; (d) light blue; (e) white.
ANS: One gene with four alleles. (a) purple: c
p
c
p
, c
p
c
b
; c
p
c
t
, c
p
c
w
;
(b) blue: c
b
c
b
, c
b
c
t
, c
b
c
w
; (c) turquoise: c
t
c
t
, c
t
c
w
; (d) light
blue: c
t
c
w
; (e) white: c
w
c
w
.
4.9 A woman who has blood type O and blood type M mar-
ries a man who has blood type AB and blood type MN. If
we assume that the genes for the A-B-O and M-N blood-
typing systems assort independently, what blood types
might the children of this couple have, and in what
proportions?
ANS: The woman is ii L
M
L
M
; the man is I
A
I
B
L
M
L
N
; the blood
types of the children will be A and M, A and MN, B and
M, and B and MN, all equally likely.
4.10 A Japanese strain of mice has a peculiar, uncoordinated
gait called waltzing, which is due to a recessive allele, v.
The dominant allele V causes mice to move in a coordi-
nated manner. A mouse geneticist has recently isolated
another recessive mutation that causes uncoordinated
movement. This mutation, called tango, could be an allele
of the waltzing gene, or it could be a mutation in an
entirely different gene. Propose a test to determine
whether the waltzing and tango mutations are alleles, and
if they are, propose symbols to denote them.
ANS: Cross homozygous waltzing with homozygous tango. If
the mutations are alleles, all the offspring will have an
uncoordinated gait; if they are not alleles, all the off-
spring will be wild-type. If the two mutations are alleles,
they could be denoted with the symbols v (waltzing) and
v
t
(tango).
4.11 Congenital deafness in human beings is inherited as a
recessive condition. In the following pedigree, two deaf
individuals, each presumably homozygous for a recessive
mutation, have married and produced four children with
normal hearing. Propose an explanation.
I
II
III
IV
ANS: The individuals III-4 and III-5 must be homozygous for
recessive mutations in different genes; that is, one is aa
BB and the other is AA bb; none of their children is deaf
because all of them are heterozygous for both genes
(Aa Bb).
4.12 In the fruit y, recessive mutations in either of two inde-
pendently assorting genes, brown and purple, prevent the
synthesis of red pigment in the eyes. Thus, homozygotes
for either of these mutations have brownish-purple eyes.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 9 8/14/2015 6:42:41 PM
10-WC Answers to All Questions and Problems
However, heterozygotes for both of these mutations
have dark red, that is, wild-type eyes. If such double het-
erozygotes are intercrossed, what kinds of progeny will
be produced, and in what proportions?
ANS: 9/16 dark red, 7/16 brownish purple.
4.13 The dominant mutation Plum in the fruit y also causes
brownish-purple eyes. Is it possible to determine by
genetic experiments whether Plum is an allele of the
brown or purple genes?
ANS: No. The test for allelism cannot be performed with dom-
inant mutations.
4.14 From information given in the chapter, explain why mice
with yellow coat color are not true-breeding.
ANS: The allele for yellow fur is homozygous lethal.
4.15 A couple has four children. Neither the father nor the
mother is bald; one of the two sons is bald, but neither of
the daughters is bald.
(a) If one of the daughters marries a nonbald man and
they have a son, what is the chance that the son will
become bald as an adult?
(b) If the couple has a daughter, what is the chance that
she will become bald as an adult?
ANS: The mother is Bb and the father is bb. The chance that a
daughter is Bb is 1/2. (a) The chance that the daughter
will have a bald son is (1/2) (1/2) 1/4. (b) The chance
that the daughter will have a bald daughter is zero.
4.16 The following pedigree shows the inheritance of ataxia,
a rare neurological disorder characterized by uncoordi-
nated movements. Is ataxia caused by a dominant or a
recessive allele? Explain.
I
II
III
IV
ANS: Dominant. The condition appears in every generation
and nearly every affected individual has an affected par-
ent. The exception, IV-2, had a father who carried the
ataxia allele but did not manifest the trait—an example of
incomplete penetrance.
4.17 Chickens that carry both the alleles for rose comb (R)
and pea comb (P) have walnut combs, whereas chickens
that lack both of these alleles (i.e., they are genotypically
rr pp) have single combs. From the information about
interactions between these two genes given in the chap-
ter, determine the phenotypes and proportions expected
from the following crosses:
(a) RR Pp rr Pp;
(b) rr PP Rr Pp;
(c) Rr Pp Rr pp;
(d) Rr pp rr pp.
ANS: (a) 3/4 walnut, 1/4 rose; (b) 1/2 walnut, 1/2 pea; (c) 3/8
walnut, 3/8 rose, 1/8 pea, 1/8 single; (d) 1/2 rose, 1/2
single.
4.18 Rose-comb chickens mated with walnut-comb chickens
produced 15 walnut-, 14 rose-, 5 pea-, and 6 single-comb
chicks. Determine the genotypes of the parents.
ANS: Rr pp Rr Pp.
4.19 Summer squash plants with the dominant allele C bear
white fruit, whereas plants homozygous for the recessive
allele c bear colored fruit. When the fruit is colored, the
dominant allele G causes it to be yellow; in the absence
of this allele (i.e., with genotype gg), the fruit color is
green. What are the F
2
phenotypes and proportions
expected from intercrossing the progeny of CC GG and
cc gg plants? Assume that the C and G genes assort
independently.
ANS: 12/16 white, 3/16 yellow, 1/16 green.
4.20 The white Leghorn breed of chickens is homozygous for
the dominant allele C, which produces colored feathers.
However, this breed is also homozygous for the domi-
nant allele I of an independently assorting gene that
inhibits coloration of the feathers. Consequently, Leg-
horn chickens have white feathers. The white Wyandotte
breed of chickens has neither the allele for color nor the
inhibitor of color; it is therefore genotypically cc ii. What
are the F
2
phenotypes and proportions expected from
intercrossing the progeny of a white Leghorn hen and a
white Wyandotte rooster?
ANS: 13/16 white, 3/16 colored.
4.21 Fruit ies homozygous for the recessive mutation scarlet
have bright red eyes because they cannot synthesize
brown pigment. Fruit ies homozygous for the recessive
mutation brown have brownish-purple eyes because they
cannot synthesize red pigment. Fruit ies homozygous
for both of these mutations have white eyes because they
cannot synthesize either type of pigment. The brown and
scarlet mutations assort independently. If fruit ies that
are heterozygous for both of these mutations are inter-
crossed, what kinds of progeny will they produce, and in
what proportions?
ANS: 9/16 dark red (wild-type), 3/16 brownish purple, 3/16
bright red, 1/16 white.
4.22 Consider the following hypothetical scheme of determi-
nation of coat color in a mammal. Gene A controls the
conversion of a white pigment P
0
into a gray pigment
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 10 8/14/2015 6:42:42 PM

Answers to All Questions and Problems WC-11
P
1
; the dominant allele A produces the enzyme necessary
for this conversion, and the recessive allele a produces an
enzyme without biochemical activity. Gene B controls
the conversion of the gray pigment P
1
into a black pig-
ment P
2
; the dominant allele B produces the active
enzyme for this conversion, and the recessive allele b
produces an enzyme without activity. The dominant
allele C of a third gene produces a polypeptide that com-
pletely inhibits the activity of the enzyme produced by
gene A; that is, it prevents the reaction P
0
→P
1
. Allele c of
this gene produces a defective polypeptide that does not
inhibit the reaction P
0
→P
1
. Genes A, B, and C assort
independently, and no other genes are involved. In the F
2
of the cross AA bb CC aa BB cc, what is the expected
phenotypic segregation ratio?
ANS: 9 black: 3 gray: 52 white.
4.23 What F
2
phenotypic segregation ratio would be expected
for the cross described in the preceding problem if the
dominant allele, C, of the third gene produced a product
that completely inhibited the activity of the enzyme pro-
duced by gene B—that is, prevented the reaction P
1
→P
2
,
rather than inhibiting the activity of the enzyme pro-
duced by gene A?
ANS: 9 black: 39 gray: 16 white.
4.24 The Micronesian Kingsher, Halcyon cinnamomina, has a
cinnamon-colored face. In some birds, the color contin-
ues onto the chest, producing one of three patterns: a
circle, a shield, or a triangle; in other birds, there is no
color on the chest. A male with a colored triangle was
crossed with a female that had no color on her chest, and
all their offspring had a colored shield on the chest.
When these offspring were intercrossed, they produced
an F
2
with a phenotypic ratio of 3 circle: 6 shield: 3 tri-
angle: 4 no color. (a) Determine the mode of inheritance
for this trait and indicate the genotypes of the birds in all
three generations. (b) If a male without color on his chest
is mated to a female with a colored shield on her chest
and the F
1
segregate in the ratio of 1 circle: 2 shield:
1 triangle, what are the genotypes of the parents and
their progeny?
(a) The simplest explanation for the inheritance of the
trait is recessive epistasis combined with incomplete
dominance, summarized in the following table:
Genotype Phenotype Frequency in F
2
AA B- Circle 3/16
Aa B- Shield 6/16
aa B- Triangular 3/16
A- bb No color 3/16
aa bb No color 1/16
(b) Father’s genotype: Aa bb; mother’s genotype: Aa BB
Circle Shield Triangle
Progeny
genotypes:
AA Bb Aa Bb aa Bb
4.25 In a species of tree, seed color is determined by four
independently assorting genes: A, B, C, and D. The
recessive alleles of each of these genes (a, b, c, and d) pro-
duce abnormal enzymes that cannot catalyze a reaction
in the biosynthetic pathway for seed pigment. This path-
way is diagrammed as follows:
White precursor
Yellow Orange
AB
Red
Blue
C
D
When both red and blue pigments are present, the seeds
are purple. Trees with the genotypes Aa Bb Cc Dd and Aa
Bb Cc dd were crossed.
(a) What color are the seeds in these two parental
genotypes?
(b) What proportion of the offspring from the cross will
have white seeds?
(c) Determine the relative proportions of red, white, and
blue offspring from the cross.
ANS: (a) Purple red; (b) proportion white (aa) 1/4;
(c) proportion red (A- B- C- dd) (3/4)(3/4)(3/4)(1/2)
27/128, proportion white (aa) 1/4 32/128, propor-
tion blue (A- B- cc Dd) (3/4)(3/4)(1/4)(1/2) 9/128.
4.26 Multiple crosses were made between true-breeding lines
of black and yellow Labrador retrievers. All the F
1
prog-
eny were black. When these progeny were intercrossed,
they produced an F
2
consisting of 91 black, 39 yellow,
and 30 chocolate. (a) Propose an explanation for the
inheritance of coat color in Labrador retrievers. (b) Pro-
pose a biochemical pathway for coat color determination
and indicate how the relevant genes control coat
coloration.
ANS: (a) Because the F
2
segregation is approximately 9 black:
3 chocolate: 4 yellow, coat color is determined by epista-
sis between two independently assorting genes: black
B- E-; chocolate bb E-; yellow B- ee or bb ee.
(b) Yellow pigment—E brown pigment—B black
pigment.
4.27 Two plants with white owers, each from true-breeding
strains, were crossed. All the F
1
plants had red owers.
When these F
1
plants were intercrossed, they produced
an F
2
consisting of 177 plants with red owers and
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 11 8/14/2015 6:42:42 PM
12-WC Answers to All Questions and Problems
142 with white owers. (a) Propose an explanation for
the inheritance of ower color in this plant species.
(b) Propose a biochemical pathway for ower pigmenta-
tion and indicate which genes control which steps in this
pathway.
ANS: (a) Because the F
2
segregation is approximately 9 red: 7
white, ower color is due to epistasis between two inde-
pendently assorting genes: red A- B- and white aa
B-, A- bb, or aa bb. (b) Colorless precursor—A colorless
product—B red pigment.
4.28 Consider the following genetically controlled biosyn-
thetic pathway for pigments in the owers of a hypo-
thetical plant:
P
0
Gene A
Enzyme A
P
1
Gene B
Enzyme B
P
2
P
3
Gene C
Enzyme C
Assume that gene A controls the conversion of a white
pigment, P
0
, into another white pigment, P
1
; the
dominant allele A species an enzyme necessary for
this conversion, and the recessive allele a species a
defective enzyme without biochemical function. Gene
B controls the conversion of the white pigment, P
1
,
into a pink pigment, P
2
; the dominant allele, B, pro-
duces the enzyme necessary for this conversion, and
the recessive allele, b, produces a defective enzyme.
The dominant allele, C, of the third gene species an
enzyme that converts the pink pigment, P
2
, into a red
pigment, P
3
; its recessive allele, c, produces an altered
enzyme that cannot carry out this conversion. The
dominant allele, D, of a fourth gene produces a poly-
peptide that completely inhibits the function of
enzyme C; that is, it blocks the reaction P
2
→P
3
. Its
recessive allele, d, produces a defective polypeptide
that does not block this reaction. Assume that ower
color is determined solely by these four genes and that
they assort independently. In the F
2
of a cross between
plants of the genotype AA bb CC DD and plants of the
genotype aa BB cc dd, what proportion of the plants
will have (a) red owers? (b) pink owers? (c) white
owers?
ANS: (a) Proportion red (3/4)
3
(1/4) 27/256; (b) pro-
portion pink (3/4)
4
[(3/4)
2
(1/4)] 117/256; (c)
proportion white 1 144/256 112/256.
4.29 In the following pedigrees, what are the inbreeding coef-
cients of A, B, and C?
AB
Offspring of first
cousins once
removed
C
Offspring of second
cousins
Offspring of half–first
cousins
ANS: F
A
(1/2)
5
1/32; F
B
2 (1/2)
6
1/32; F
C
2
(1/2)
7
1/64.
4.30 A, B, and C are inbred strains of mice, assumed to be
completely homozygous. A is mated to B and B to C.
Then the A B hybrids are mated to C, and the off-
spring of this mating are mated to the B C hybrids.
What is the inbreeding coefcient of the offspring of this
last mating?
ANS: From the following pedigree, the inbreeding coefcient
is (1/2)
3
(1 F
C
) (1/2)
4
(1 F
B
) 3/8 because F
B
F
C
1.
A
A ×
BB
× C
(A × B) × C
BC
4.31 Mabel and Frank are half siblings, as are Tina and Tim.
However, these two pairs of half siblings do not have any
common ancestors. If Mabel marries Tim and Frank
marries Tina and each couple has a child, what fraction
of their genes will these children share by virtue of
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 12 8/14/2015 6:42:43 PM
Answers to All Questions and Problems WC-13
common ancestry? Will the children be more or less
closely related than rst cousins?
ANS: The pedigree is shown below:
Frank
Mabel Tina
Tim
The coefcient of relationship between the offspring of
the two couples is obtained by calculating the inbreeding
coefcient of the imaginary child from a mating between
these offspring and multiplying by 2: [(1/2)
5
2] 2
1/8. This is the same degree of relatedness as rst
cousins.
4.32 Suppose that the inbreeding coefcient of I in the fol-
lowing pedigree is 0.25. What is the inbreeding coef-
cient of I’s common ancestor, C?
I
C
ANS: F
I
(1/2)
3
(1 F
C
) 0.25; thus, F
C
1.
4.33 A randomly pollinated strain of maize produces ears that
are 24 cm long, on average. After one generation of self-
fertilization, the ear length is reduced to 20 cm. Predict
the ear length if self-fertilization is continued for one
more generation.
ANS: The mean ear length for randomly mated maize is
24 cm and that for maize from one generation of self-
fertilization is 20 cm. The inbreeding coefcient of the
offspring of one generation of self-fertilization is 1/2,
and the inbreeding coefcient of the offspring of two
generations of self-fertilization is (1/2)(11/2) 3/4.
Mean ear length (Y ) is expected to decline linearly with
inbreeding according to the equation Y 24 b F
1
where b is the slope of the line. The value of b can be
determined from the two values of Y that are given. The
difference between these two values (4 cm) corresponds
to an increase in F from 0 to 1/2. Thus, b 4/(1/2)
8 cm, and for F 3/4, the predicted mean ear length is
Y 24 8 (3/4) 18 cm.
Chapter 5
5.1 What are the genetic differences between male- and
female-determining sperm in animals with heteroga-
metic males?
ANS: The male-determining sperm carries a Y chro mo-
some; the female-determining sperm carries an X
chromosome.
5.2 A male with singed bristles appeared in a culture of Dro-
sophila. How would you determine if this unusual pheno-
type was due to an X-linked mutation?
ANS: Cross the singed male to wild-type females and then
intercross the offspring. If the singed bristle phenotype is
due to an X-linked mutation, approximately half the F
2
males, but none of the F
2
females, will show it.
5.3 In grasshoppers, rosy body color is caused by a recessive
mutation; the wild-type body color is green. If the gene
for body color is on the X chromosome, what kind of
progeny would be obtained from a mating between a
homozygous rosy female and a hemizygous wild-type
male? (In grasshoppers, females are XX and males are
XO.)
ANS: All the daughters will be green and all the sons will be
rosy.
5.4 In the mosquito Anopheles culicifacies, golden body (go) is a
recessive X-linked mutation, and brown eyes (bw) is a
recessive autosomal mutation. A homozygous XX female
with golden body is mated to a homozygous XY male
with brown eyes. Predict the phenotypes of their F
1
offspring. If the F
1
progeny are intercrossed, what kinds
of progeny will appear in the F
2
and in what
proportions?
ANS: The cross is go/go / female /Y bw/bw male → F
1
:
go/ bw/ females (wild-type eyes and body) and go/Y
bw/ males (golden body, wild-type eyes). An intercross
of the F
1
offspring yields the following F
2
phenotypes in
both sexes.
Body Eyes Genotype Proportion
Golden Brown go/go or Y bw/bw
(1/2) (1/4)
1/8
Golden Wild-
type
go/go or Y
/bw
or
(1/2) (3/4)
3/8
Wild-
type
Brown
/go or Y
bw/bw
(1/2) (1/4)
1/8
Wild-
type
Wild-
type
/go or Y /bw
or
(1/2) (3/4)
3/8
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 13 8/14/2015 6:42:43 PM
14-WC Answers to All Questions and Problems
5.5 What are the sexual phenotypes of the following geno-
types in Drosophila: XX, XY, XXY, XXX, XO?
ANS: XX is female, XY is male, XXY is female, XXX is female
(but barely viable), XO is male (but sterile).
5.6 In human beings, a recessive X-linked mutation, g, causes
green-defective color vision; the wild-type allele, G,
causes normal color vision. A man (a) and a woman (b),
both with normal vision, have three children, all married
to people with normal vision: a color-defective son (c),
who has a daughter with normal vision (f); a daughter
with normal vision (d), who has one color-defective son
(g) and two normal sons (h); and a daughter with normal
vision (e), who has six normal sons (i). Give the most
likely genotypes for the individuals (a–i) in this family.
(a)
(c)
(f)
(i) 6
(d) (e)
(b)
(h)
(g)
G/Y
g/Y
G/Y G/Y
g/Y
G/g
g/G G/G
G/g
ANS: (a) X
G
Y; (b) X
G
X
g
; (c) X
g
Y; (d) X
G
X
g
; (e) X
G
X
G
; (f) X
G
X
g
;
(g) X
g
Y; (h) X
G
Y; (i) X
G
Y
5.7 If both father and son have defective color vision, is it
likely that the son inherited the trait from his father?
ANS: No. Defective color vision is caused by an X-linked
mutation. The son’s X chromosome came from his
mother, not his father.
5.8 A normal woman, whose father had hemophilia, marries
a normal man. What is the chance that their rst child
will have hemophilia?
ANS: The risk for the child is P(woman transmits mutant
allele) P(child is male) (1/2) (1/2) 1/4.
5.9 A man with X-linked color blindness marries a woman
with no history of color blindness in her family. The
daughter of this couple marries a normal man, and their
daughter also marries a normal man. What is the chance
that this last couple will have a child with color blindness?
If this couple has already had a child with color blindness,
what is the chance that their next child will be color blind?
ANS: The risk for the child is P(mother is C/c) P(mother
transmits c) P(child is male) (1/2) (1/2) (1/2)
1/8; if the couple has already had a child with color
blindness, P(mother is C/c) 1, and the risk for each
subsequent child is 1/4.
5.10 A man who has color blindness and type O blood has
children with a woman who has normal color vision and
type AB blood. The woman’s father had color blindness.
Color blindness is determined by an X-linked gene, and
blood type is determined by an autosomal gene.
(a) What are the genotypes of the man and the woman?
(b) What proportion of their children will have color
blindness and type B blood?
(c) What proportion of their children will have color
blindness and type A blood?
(d) What proportion of their children will be color blind
and have type AB blood?
ANS: (a) The man is X
c
Y ii; the woman is X
X
c
I
A
I
B
. (b) Prob-
ability color blind 1/2; probability type B blood 1/2;
combined probability (1/2) (1/2) 1/4. (c) Proba-
bility color blind 1/2; probability type A blood 1/2;
combined probability (1/2) (1/2) 1/4. (d) 0.
5.11 A Drosophila female homozygous for a recessive X-linked
mutation that causes vermilion eyes is mated to a wild-
type male with red eyes. Among their progeny, all the
sons have vermilion eyes, and nearly all the daughters
have red eyes; however, a few daughters have vermilion
eyes. Explain the origin of these vermilion-eyed
daughters.
ANS: Each of the rare vermilion daughters must have resulted
from the union of an X(v) X(v) egg with a Y-bearing
sperm. The diplo-X eggs must have originated through
nondisjunction of the X chromosomes during oogenesis
in the mother. However, we cannot determine if the
nondisjunction occurred in the rst or the second mei-
otic division.
5.12 In Drosophila, vermilion eye color is due to a recessive
allele (v) located on the X chromosome. Curved wings
are due to a recessive allele (cu) located on one autosome,
and ebony body is due to a recessive allele (e) located on
another autosome. A vermilion male is mated to a curved,
ebony female, and the F
1
males are phenotypically wild-
type. If these males were backcrossed to curved, ebony
females, what proportion of the F
2
offspring will be wild-
type males?
ANS: P(male) 1/2; P(male transmits rst wild-type autosome)
1/2; P(male transmits other wild-type autosome) 1/2;
therefore, combined proportion, P(wild-type male) 1/8
5.13 A Drosophila female heterozygous for the recessive
X-linked mutation w (for white eyes) and its wild-type
allele w
1
is mated to a wild-type male with red eyes.
Among the sons, half have white eyes and half have red
eyes. Among the daughters, nearly all have red eyes;
however, a few have white eyes. Explain the origin of
these white-eyed daughters.
ANS: Each of the rare white-eyed daughters must have resulted
from the union of an X(w) X(w) egg with a Y-bearing
sperm. The rare diplo-X eggs must have originated
through nondisjunction of the X chromosomes during
the second meiotic division in the mother.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 14 8/14/2015 6:42:44 PM

Answers to All Questions and Problems WC-15
5.14 In Drosophila, a recessive mutation called chocolate (c)
causes the eyes to be darkly pigmented. The mutant phe-
notype is indistinguishable from that of an autosomal
recessive mutation called brown (bw). A cross of choco-
late-eyed females to homozygous brown males yielded
wild-type F
1
females and darkly pigmented F
1
males. If
the F
1
ies are intercrossed, what types of progeny are
expected, and in what proportions? (Assume that the
double mutant combination has the same phenotype as
either of the single mutants alone.)
ANS: 3/8 wild-type (red), 5/8 brown for both male and female
F
2
progeny.
5.15 Suppose that a mutation occurred in the SRY gene on the
human Y chromosome, knocking out its ability to pro-
duce the testis-determining factor. Predict the pheno-
type of an individual who carried this mutation and a
normal X chromosome.
ANS: Female.
5.16 A woman carries the androgen-insensitivity mutation
(ar) on one of her X chromosomes; the other X carries
the wild-type allele (AR). If the woman marries a normal
man, what fraction of her children will be phenotypically
female? Of these, what fraction will be fertile?
ANS: Three-fourths will be phenotypically female (genotypi-
cally ar/AR, AR/AR, or ar/Y). Among the females, 2/3
(ar/AR and AR/AR) will be fertile; the ar/Y females will
be sterile.
5.17 Would a human with two X chromosomes and a Y chro-
mosome be male or female?
ANS: Male.
5.18 In Drosophila, the gene for bobbed bristles (recessive allele
bb, bobbed bristles; wild-type allele
, normal bristles) is
located on the X chromosome and on a homologous seg-
ment of the Y chromosome. Give the genotypes and
phenotypes of the offspring from the following crosses:
(a) X
bb
X
bb
X
bb
Y
;
(b) X
bb
X
bb
X
bb
Y
;
(c) X
X
bb
X
Y
bb
;
(d) X
X
bb
X
bb
Y
.
ANS: (a) 1/2 X
bb
X
bb
bobbed females, 1/2 X
bb
Y
wild-type
males; (b) 1/2 X
X
bb
wild-type females, 1/2 X
bb
Y
bb
bobbed males; (c) 1/4 X
X
wild-type females, 1/4 X
X
bb
wild-type females, 1/4 X
Y
bb
wild-type males, 1/4 X
bb
Y
bb
bobbed males; (d) 1/4 X
X
bb
wild-type females, 1/4
X
bb
X
bb
bobbed females, 1/4 X
Y
wild-type males, 1/4
X
bb
Y
wild-type males.
5.19 Predict the sex of Drosophila with the following chromo-
some compositions (A = haploid set of autosomes):
(a) 4X 4A;
(b) 3X 4A;
(c) 2X 3A;
(d) 1X 3A;
(e) 2X 2A;
(f) 1X 2A.
ANS: (a) Female; (b) intersex; (c) intersex; (d) male: (e) female;
(f) male.
5.20 In chickens, the absence of barred feathers is due to a
recessive allele. A barred rooster was mated with a non-
barred hen, and all the offspring were barred. These F
1
chickens were intercrossed to produce F
2
progeny,
among which all the males were barred; half the females
were barred and half were nonbarred. Are these results
consistent with the hypothesis that the gene for barred
feathers is located on one of the sex chromosomes?
ANS: Yes. The gene for feather patterning is on the Z chromo-
some. If we denote the allele for barred feathers as B and
the allele for nonbarred feathers as b, the crosses are as
follows: B/B (barred) male b/W (nonbarred) female →
F
1
: B/b (barred) males and B/W (barred) females. Inter-
crossing the F
1
produces B/B (barred) males, B/b (barred)
males, B/W (barred) females, and b/W (nonbarred)
females, all in equal proportions.
5.21 A Drosophila male carrying a recessive X-linked mutation
for yellow body is mated to a homozygous wild-type
female with gray body. The daughters of this mating all
have uniformly gray bodies. Why are not their bodies a
mosaic of yellow and gray patches?
ANS: Drosophila does not achieve dosage compensation by
inactivating one of the X chromosomes in females.
5.22 What is the maximum number of Barr bodies in the
nuclei of human cells with the following chromosome
compositions:
(a) XY;
(b) XX;
(c) XXY;
(d) XXX;
(e) XXXX;
(f) XYY?
ANS: a) Zero; (b) one; (c) one; (d) two; (e) three; (f) zero.
5.23 Males in a certain species of deer have two nonhomolo-
gous X chromosomes, denoted X
1
and X
2
, and a Y chro-
mosome. Each X chromosome is about half as large as
the Y chromosome, and its centromere is located near
one of the ends; the centromere of the Y chromosome is
located in the middle. Females in this species have two
copies of each of the X chromosomes and lack a Y chro-
mosome. How would you predict the X and Y chromo-
somes to pair and disjoin during spermatogenesis to
produce equal numbers of male- and female-determin-
ing sperm?
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 15 8/14/2015 6:42:44 PM
16-WC Answers to All Questions and Problems
ANS:
Metaphase Anaphase
Y
X
1
X
2
Since the centromere is at the end of each small X chro-
mosome but in the middle of the larger Y, both X
1
and X
2
pair at the centromere of the Y chromosome during meta-
phase so that the two X chromosomes disjoin together
and segregate from the Y chromosome during anaphase.
5.24 A breeder of sun conures (a type of bird) has obtained
two true-breeding strains, A and B, which have red eyes
instead of the normal brown found in natural popula-
tions. In Cross 1, a male from strain A was mated to a
female from strain B, and the male and female offspring
all had brown eyes. In Cross 2, a female from strain A was
mated to a male from strain B, and the male offspring
had brown eyes and the female offspring had red eyes.
When the F
1
birds from each cross were mated brother
to sister, the breeder obtained the following results:
Phenotype
Proportion in
F
2
of Cross 1
Proportion in
F
2
of Cross 2
Brown male 6/16 3/16
Red male 3/16 5/16
Brown female 3/16 3/16
Red female 5/16 5/16
Provide a genetic explanation for these results.
ANS: Color is determined by an autosomal gene (alleles A
and a) and a sex-linked gene (alleles B and b) on the Z
chromosome (females are ZW and males are ZZ) and
the recessive alleles are mutually epistatic—that is, aa, bb,
or bW birds have red eyes, and A- B- or A- BW birds
have brown eyes.
Cross 1
P Strain A red male
Strain B red female
aa BB AA bW
F
1
Aa Bb
Brown males
Aa BW
Brown females
F
2
A- Bb
Brown males (6/16)
A- BW
Brown females (3/16)
aa Bb
Red males (2/16)
A- bW
Red females (4/16)
aa BW
Red females (1/16)
Cross 2
P Strain A red female
Strain B red male
aa BW AA bb
F
1
Aa Bb
Brown males
Aa bW
Red females
F
2
A- Bb
Brown males (3/16)
A- bW
Brown females (3/16)
A- bb
Red males (3/16)
A- bW
Red females (3/16)
aa b-
Red males 2/16
aa -W
Red females (2/16)
5.25 In 1908, F. M. Durham and D. C. E. Marryat reported
the results of breeding experiments with canaries. Cin-
namon canaries have pink eyes when they rst hatch,
whereas green canaries have black eyes. Durham and
Marryat crossed cinnamon females with green males
and observed that all the F
1
progeny had black eyes,
just like those of the green strain. When the F
1
males
were crossed to green females, all the male progeny
had black eyes, whereas all the female progeny had
either black or pink eyes, in about equal proportions.
When the F
1
males were crossed to cinnamon females,
four classes of progeny were obtained: females with
black eyes, females with pink eyes, males with black
eyes, and males with pink eyes—all in approximately
equal proportions. Propose an explanation for these
ndings.
ANS: Eye color in canaries is due to a gene on the Z chromo-
some, which is present in two copies in males and one
copy in females. The allele for pink color at hatching
(p) is recessive to the allele for black color at hatching
(P). There is no eye color gene on the other sex chro-
mosome (W), which is present in one copy in females
and absent in males. The parental birds were genotypi-
cally p/W (cinnamon females) and P/P (green males).
Their F
1
sons were genotypically p/P (with black eyes
at hatching). When these sons were crossed to green
females (genotype P/W), they produced F
2
progeny
that sorted into three categories: males with black eyes
at hatching (P/-, half the total progeny), females with
black eyes at hatching (P/W, a fourth of the total prog-
eny), and females with pink eyes at hatching (p/W, a
fourth of the total progeny). When these sons were
crossed to cinnamon females (genotype p/W), they
produced F
2
progeny that sorted into four equally fre-
quent categories: males with black eyes at hatching
(genotype P/p), males with pink eyes at hatching (gen-
otype p/p), females with black eyes at hatching
(genotype P/W), and females with pink eyes at hatch-
ing (genotype p/W).
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 16 8/14/2015 6:42:44 PM

Answers to All Questions and Problems WC-17
Chapter 6
6.1 In the human karyotype, the X chromosome is approxi-
mately the same size as seven of the autosomes (the
so-called C group of chromosomes). What procedure
could be used to distinguish the X chromosome from the
other members of this group?
ANS: Use one of the banding techniques.
6.2 In humans, a cytologically abnormal chromosome 22,
called the “Philadelphia” chromosome because of the
city in which it was discovered, is associated with
chronic leukemia. This chromosome is missing part of
its long arm. How would you denote the karyotype of
an individual who had 46 chromosomes in his somatic
cells, including one normal 22 and one Philadelphia
chromosome?
ANS: 46, XX, del(22)(q) or 46, XY, del(22)(q), depending on
the sex chromosome constitution.
6.3 During meiosis, why do some tetraploids behave more
regularly than triploids?
ANS: In allotetraploids, each member of the different sets
of chromosomes can pair with a homologous partner
during prophase I and then disjoin during anaphase I.
In triploids, disjunction is irregular because homol o-
gous chromosomes associate during prophase I either
by forming bivalents and univalents or by forming
trivalents.
6.4 The following table presents chromosome data on four
species of plants and their F
1
hybrids:
Meiosis I Metaphase
Species or
F
1
Hybrid
Root Tip
Chromosome
Number
Number of
Bivalents
Number of
Univalents
A 20 10 0
B 20 10 0
C 10 5 0
D 10 5 0
A B
20 0 20
A C
15 5 5
A D
15 5 5
C D
10 0 10
(a) Deduce the chromosomal origin of species A.
(b) How many bivalents and univalents would you expect
to observe at meiotic metaphase I in a hybrid between
species C and species B?
(c) How many bivalents and univalents would you expect
to observe at meiotic metaphase I in a hybrid between
species D and species B?
ANS: (a) Species A is an allotetraploid with a genome from
each of species C and species D; (b) 0 bivalents and
15univalents; (c) 0 bivalents and 15 univalents.
6.5 A plant species A, which has seven chromosomes in its
gametes, was crossed with a related species B, which has
nine. The hybrids were sterile, and microscopic observa-
tion of their pollen mother cells showed no chromosome
pairing. A section from one of the hybrids that grew vig-
orously was propagated vegetatively, producing a plant
with 32 chromosomes in its somatic cells. This plant was
fertile. Explain.
ANS: The fertile plant is an allotetraploid with 7 pairs of chro-
mosomes from species A and 9 pairs of chromosomes
from species B; the total number of chromosomes is
(2 7) (2 9) 32.
6.6 A plant species X with n 5 was crossed with a related
species Y with n 7. The F
1
hybrid produced only a few
pollen grains, which were used to fertilize the ovules of
species Y. A few plants were produced from this cross,
and all had 19 chromosomes. Following self-fertilization,
the F
1
hybrids produced a few F
2
plants, each with
24chromosomes. These plants were phenotypically dif-
ferent from either of the original species and were highly
fertile. Explain the sequence of events that produced
these fertile F
2
hybrids.
ANS: The F
1
hybrid had 5 chromosomes from species X and 7
chromosomes from species Y, for a total of 12. When this
hybrid was backcrossed to species Y, the few progeny
that were produced had 5 7 12 chromosomes from
the hybrid and 7 from species Y, for a total of 19. This
hybrid was therefore a triploid. Upon self-fertilization,
a few F
2
plants were formed, each with 24 chromosomes.
Presumably the chromosomes in these plants consisted
of 2 5 10 from species X and 2 7 14 from spe-
cies Y. These vigorous and fertile F
2
plants were there-
fore allotetraploids.
6.7 Identify the sexual phenotypes of the following
genotypes in human beings: XX, XY, XO, XXX, XXY,
XYY.
ANS: XX is female, XY is male, XO is female (but sterile), XXX
is female, XXY is male (but sterile), and XYY is male.
6.8 If nondisjunction of chromosome 21 occurs in the divi-
sion of a secondary oocyte in a human female, what is the
chance that a mature egg derived from this division will
receive two number 21 chromosomes?
ANS: 1/2
6.9 A Drosophila female homozygous for a recessive X-linked
mutation causing yellow body was crossed to a wild-type
male. Among the progeny, one y had sectors of yellow
pigment in an otherwise gray body. These yellow sectors
were distinctly male, whereas the gray areas were female.
Explain the peculiar phenotype of this y.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 17 8/14/2015 6:42:44 PM

18-WC Answers to All Questions and Problems
ANS: The y is a gynandromorph, that is, a sexual mosaic. The
yellow tissue is X(y)/O and the gray tissue is X(y)/X().
This mosaicism must have arisen through loss of the
X chromosome that carried the wild-type allele, pre-
sumably during one of the early embryonic cleavage
divisions.
6.10 The Drosophila fourth chromosome is so small that ies
monosomic or trisomic for it survive and are fertile. Sev-
eral genes, including eyeless (ey), have been located on this
chromosome. If a cytologically normal y homozygous
for a recessive eyeless mutation is crossed to a y mono-
somic for a wild-type fourth chromosome, what kinds of
progeny will be produced, and in what proportions?
ANS: Approximately half the progeny should be disomic ey/
and half should be monosomic ey/O. The disomic prog-
eny will be wild-type, and the monosomic progeny will
be eyeless.
6.11 A woman with X-linked color blindness and Turner syn-
drome had a color-blind father and a normal mother. In
which of her parents did nondisjunction of the sex chro-
mosomes occur?
ANS: Nondisjunction must have occurred in the mother. The
color blind woman with Turner syndrome was produced
by the union of an X-bearing sperm, which carried the
mutant allele for color blindness, and a nullo-X egg.
6.12 In humans, Hunter syndrome is known to be an X-linked
trait with complete penetrance. In family A, two pheno-
typically normal parents have produced a normal son,
a daughter with Hunter and Turner syndromes, and a son
with Hunter syndrome. In family B, two phenotypically
normal parents have produced two phenotypically nor-
mal daughters and a son with Hunter and Klinefelter syn-
dromes. In family C, two phenotypically normal parents
have produced a phenotypically normal daughter,
a daughter with Hunter syndrome, and a son with Hunter
syndrome. For each family, explain the origin of the child
indicated in italics.
ANS: The daughter with Turner and Hunter syndromes in
family A must have received her single X chromosome
from her mother, who is heterozygous for the mutation
causing Hunter syndrome. The daughter did not receive
a sex chromosome from her father because sex chromo-
some nondisjunction must have occurred during meiosis
in his germline. The son with Klinefelter syndrome in
family B is karyotypically XXY, and both of his X chro-
mosomes carry the mutant allele for Hunter syndrome.
This individual must have received two mutant
X chromosomes from his heterozygous mother due to X
chromosome nondisjunction during the second meiotic
division in her germline. The daughter with Hunter syn-
drome in family C is karyotypically XX, and both of her
X chromosomes carry the mutant allele for Hunter syn-
drome. This individual received the two mutant X
chromosomes from her heterozygous mother through
nondisjunction during the second meiotic division in the
mother’s germline. Furthermore, because the daughter
did not receive a sex chromosome from her father, sex
chromosome nondisjunction must have occurred during
meiosis in his germline too.
6.13 Although XYY men are phenotypically normal, would
they be expected to produce more children with sex
chromosome abnormalities than XY men? Explain.
ANS: XYY men would produce more children with sex chro-
mosome abnormalities because their three sex chromo-
somes will disjoin irregularly during meiosis. This
irregular disjunction will produce a variety of aneuploid
gametes, including the XY, YY, XYY, and nullo sex chro-
mosome constitutions.
6.14 In a Drosophila salivary chromosome, the bands have a
sequence of 1 2 3 4 5 6 7 8. The homologue with which
this chromosome is synapsed has a sequence of 1 2 3 6 5
4 7 8. What kind of chromosome change has occurred?
Draw the synapsed chromosomes.
ANS: The animal is heterozygous for an inversion:
2
1
3
2
1
3
66 44
7
8
7
8
5
5
6.15 Other chromosomes have sequences as follows: (a) 1 2 5
6 7 8; (b) 1 2 3 4 4 5 6 7 8; (c) 1 2 3 4 5 8 7 6. What kind
of chromosome change is present in each? Illustrate how
these chromosomes would pair with a chromosome
whose sequence is 1 2 3 4 5 6 7 8.
ANS: (a) Deletion:
12 5678
12
34
34
5678
(b) Duplication:
1 234 56 87
1
234
4
4
56
8
7
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 18 8/14/2015 6:42:45 PM
Answers to All Questions and Problems WC-19
(c) A terminal inversion:
12345
68
7
1234
6
7
8
5
6.16 In plants translocation heterozygotes display about 50 per-
cent pollen abortion. Why?
ANS: In translocation heterozygotes, only alternate segrega-
tion leads to euploid gametes, and the frequency of alter-
nate segregation is typically around 5 percent.
6.17 One chromosome in a plant has the sequence A B C D E
F, and another has the sequence M N O P Q R. A recip-
rocal translocation between these chromosomes pro-
duced the following arrangement: A B C P Q R on one
chromosome and M N O D E F on the other. Illustrate
how these translocated chromosomes would pair with
their normal counterparts in a heterozygous individual
during meiosis.
ANS:
AB
CONM
AB
CONM
D
E
F
D
E
F
R
Q
P
R
Q
P
6.18 In Drosophila, the genes bw and st are located on chromo-
somes 2 and 3, respectively. Flies homozygous for bw
mutations have brown eyes, ies homozygous for st
mutations have scarlet eyes, and ies homozygous for
bw and st mutations have white eyes. Doubly heterozy-
gous males were mated individually to homozygous bw;
st females. All but one of the matings produced four
classes of progeny: wild-type, and brown-, scarlet- and
white-eyed. The single exception produced only wild-
type and white-eyed progeny. Explain the nature of this
exception.
ANS: The exceptional male, whose genotype is bw/ st/, is
heterozygous for a translocation between chromosomes
2 and 3. It is not possible to determine whether the
translocation is between the two mutant chromosomes
or between the two wild-type chromosomes, that is,
whether it is T(bw; st) or T(; ); however, it clearly is
not between a mutant chromosome and a wild-type
chromosome, that is, T(bw; ) or T(; st). If it were, the
progeny would be either brown or scarlet, not either
wild-type or white.
6.19 A phenotypically normal boy has 45 chromosomes, but
his sister, who has Down syndrome, has 46. Suggest an
explanation for this paradox.
ANS: The boy carries a translocation between chromosome 21
and another chromosome, say chromosome 14. He also
carries a normal chromosome 21 and a normal chromo-
some 14. The boy’s sister carries the translocation, one
normal chromosome 14, and two normal copies of chro-
mosome 21.
6.20 Distinguish between a compound chromosome and a
Robertsonian translocation.
ANS: A compound chromosome is composed of segments
from the same pair of chromosomes, as when two X
chromosomes become attached to each other. A Rob-
ertsonian translocation involves a fusion of segments
from two different pairs of chromosomes. These seg-
ments fuse at or near the centromeres, usually with the
loss of the short arms of each of the par tic i pat ing
chromosomes.
6.21 A yellow-bodied Drosophila female with attached-X
chromosomes was crossed to a white-eyed male. Both
of the parental phenotypes are caused by X-linked
recessive mutations. Predict the phenotypes of the
progeny.
ANS: All the daughters will be yellow-bodied and all the sons
will be white-eyed.
6.22 A man has attached chromosomes 21. If his wife is cyto-
logically normal, what is the chance their rst child will
have Down syndrome?
ANS: Zygotes produced by this couple will be either trisomic
or monosomic for chromosome 21. Thus, 100 percent of
their viable children will develop Down syndrome.
6.23 Analysis of the polytene chromosomes of three popula-
tions of Drosophila has revealed three different banding
sequences in a region of the second chromosome:
Population Banding Sequence
P1 1 2 3 4 5 6 7 8 9 10
P2 1 2 3 9 8 7 6 5 4 10
P3 1 2 3 9 8 5 6 7 4 10
Explain the evolutionary relationships among these
populations.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 19 8/14/2015 6:42:45 PM
20-WC Answers to All Questions and Problems
ANS: The three populations are related by a series of
inversions:
P1 1 2 3 4 5 6 7 8 9 10
P2 1 2 3 9 8 7 6 5 4 10
P3 1 2 3 9 8 5 6 7 4 10
6.24 Each of six populations of Drosophila in different geo-
graphic regions had a specic arrangement of bands in
one of the large autosomes:
(a) 12345678
(b) 12263478
(c) 15432678
(d) 14322678
(e) 16223478
(f) 154322678
Assume that arrangement (a) is the original one. In what
order did the other arrangements most likely arise, and
what type of chromosomal aberration is responsible for
each change?
ANS: Arrangement (a) produced (c) by inversion of segment
2345; (c) produced (f) by a duplication of band 2; (f) pro-
duced (d) by a deletion of band 5; (d) produced (e) by
inversion of segment 43226; (e) produced (b) by inver-
sion of segment 622.
6.25 The following diagram shows two pairs of chromosomes
in the karyotypes of a man, a woman, and their child.
The man and the woman are phenotypically normal, but
the child (a boy) suffers from a syndrome of abnormali-
ties, including poor motor control and severe mental
impairment. What is the genetic basis of the child’s
abnormal phenotype? Is the child hyperploid or hypo-
ploid for a segment in one of his chromosomes?
Mother Father Child
ANS: The mother is heterozygous for a reciprocal transloca-
tion between the long arms of the large and small chro-
mosomes; a piece from the long arm of the large
chromosome has been broken off and attached to the
long arm of the short chromosome. The child has inher-
ited the rearranged large chromosome and the normal
small chromosome from the mother. Thus, because the
rearranged large chromosome is decient for some of its
genes, the child is hypoploid.
6.26 A male mouse that is heterozygous for a reciprocal
translocation between the X chromosome and an auto-
some is crossed to a female mouse with a normal karyo-
type. The autosome involved in the translocation
carries a gene responsible for coloration of the fur. The
allele on the male’s translocated autosome is wild-type,
and the allele on its nontranslocated autosome is
mutant; however, because the wild-type allele is domi-
nant to the mutant allele, the male’s fur is wild-type
(dark in color). The female mouse has light color in her
fur because she is homozygous for the mutant allele of
the color-determining gene. When the offspring of the
cross are examined, all the males have light fur and all
the females have patches of light and dark fur. Explain
these peculiar results.
ANS: The phenotype in the female offspring is mosaic because
one of the X chromosomes is inactivated in each of their
cells. If the translocated X is inactivated, the autosome
attached to it could also be partially inactivated by a
spreading of the inactivation process across the translo-
cation breakpoint. This spreading could therefore inacti-
vate the color-determining gene on the translocated
autosome and cause patches of tissue to be phenotypi-
cally mutant.
6.27 In Drosophila, the autosomal genes cinnabar (cn) and
brown (bw) control the production of brown and red eye
pigments, respectively. Flies homozygous for cinnabar
mutations have bright red eyes, ies homozygous for
brown mutations have brown eyes, and ies homozy-
gous for mutations in both of these genes have white
eyes. A male homozygous for mutations in the cn and bw
genes has bright red eyes because a small duplication
that carries the wild-type allele of bw (bw
) is attached
to the Y chromosome. If this male is mated to a karyo-
typically normal female that is homozygous for the cn
and bw mutations, what types of progeny will be
produced?
ANS: The sons will have bright red eyes because they will
inherit the Y chromosome with the bw
allele from their
father. The daughters will have white eyes because they
will inherit an X chromosome from their father.
6.28 In Drosophila, vestigial wing (vg), hairy body (h), and eye-
less (ey) are recessive mutations on chromosomes 2, 3,
and 4, respectively. Wild-type males that had been irradi-
ated with X rays were crossed to triply homozygous
recessive females. The F
1
males (all phenotypically wild-
type) were then testcrossed to triply homozygous reces-
sive females. Most of the F
1
males produced eight classes
of progeny in approximately equal proportions, as would
be expected if the vg, h, and ey genes assort indepen-
dently. However, one F
1
male produced only four classes
of offspring, each approximately one-fourth of the total:
(1) wild-type, (2) eyeless, (3) vestigial, hairy, and (4) ves-
tigial, hairy, eyeless. What kind of chromosome aberra-
tion did the exceptional F
1
male carry, and which
chromosomes were involved?
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 20 8/14/2015 6:42:46 PM

Answers to All Questions and Problems WC-21
ANS: A reciprocal translocation between chromosomes 2
and 3. One translocated chromosome carries the wild-
type alleles of vg and h on chromosomes 2 and 3, respec-
tively, and the other carries the recessive mutant alleles
of these genes. The chromosome that carries the ey gene
(chromosome 4) is not involved in the rearrangement.
6.29 Cytological examination of the sex chromosomes in a man
has revealed that he carries an insertional translocation. A
small segment has been deleted from the Y chromosome
and inserted into the short arm of the X chromosome; this
segment contains the gene responsible for male differen-
tiation (SRY). If this man marries a karyotypically normal
woman, what types of progeny will the couple produce?
ANS: XX zygotes will develop into males because one of their X
chromosomes carries the SRY gene that was translocated
from the Y chromosome. XY zygotes will develop into
females because their Y chromosome has lost the SRY gene.
Chapter 7
7.1 Mendel did not know of the existence of chromosomes.
Had he known, what change might he have made in his
Principle of Independent Assortment?
ANS: If Mendel had known of the existence of chromosomes, he
would have realized that the number of factors determining
traits exceeds the number of chromosomes, and he would
have concluded that some factors must be linked on the
same chromosome. Thus, Mendel would have revised the
Principle of Independent Assortment to say that factors on
different chromosomes (or far apart on the same chromo-
some) are inherited independently.
7.2 From a cross between individuals with the genotypes Cc
Dd Ee cc dd ee, 1000 offspring were produced. The class
that was C- D- ee included 351 individuals. Are the genes
c, d, and e on the same or different chromosomes? Explain.
ANS: The class represented by 351 offspring indicates that at
least two of the three genes are linked.
7.3 If a is linked to b, and b to c, and c to d, does it follow
that a recombination experiment would detect linkage
between a and d? Explain.
ANS: No. The genes a and d could be very far apart on the
same chromosome—so far apart that they recombine
freely, that is, 50 percent of the time.
7.4 Mice have 19 autosomes in their genome, each about
the same size. If two autosomal genes are chosen ran-
domly, what is the chance that they will be on the same
chromosome?
ANS: 1/19
7.5 Genes on different chromosomes recombine with a fre-
quency of 50 percent. Is it possible for two genes on the
same chromosome to recombine with this frequency?
ANS: Yes, if they are very far apart.
7.6 If two loci are 10 cM apart, what proportion of the cells
in prophase of the rst meiotic division will contain a
single crossover in the region between them?
ANS: 20%
7.7 Genes a and b are 20 cM apart. An a
b
/a
b
individual
was mated with an a b/a b individual.
(a) Diagram the cross and show the gametes produced by
each parent and the genotype of the F
1
.
(b) What gametes can the F
1
produce, and in what
proportions?
(c) If the F
1
was crossed to a b/a b individuals, what off-
spring would be expected, and in what proportions?
(d) Is this an example of the coupling or repulsion link-
age phase?
(e) If the F
1
were intercrossed, what offspring would be
expected, and in what proportions?
ANS: (a) Cross: a
b
/a
b
a b/a b. Gametes: a
b
from one
parent, a b from the other. F
1
: a
b
/a b
(b) 40% a
b
, 40% a b, 10% a
b, 10% a b
(c) F
2
from testcross: 40% a
b
/a b, 40% a b/a b, 10% a
b/a b, 10% a b
/a b
(d) Coupling linkage phase
(e) F
2
from intercross:
Sperm
40% a
+
b
+
a
+
b
+
/a
+
b
+
a
+
b
+
/a
+
ba
+
b
+
/a b
+
a
+
b
+
/a b
a b/a
+
b
+
a b/a
+
ba b/a b
+
a b/a b
a
+
b/a
+
b
+
a
+
b/a
+
ba
+
b/a b
+
a
+
b/a b
a b
+
/a
+
b
+
a b
+
/a
+
ba b
+
/a b
+
a b
+
/a b
10% a b
+
10% a
+
b 10% a b
+
40% a
+
b
+
10% a
+
b
16% 16% 4% 4%
40% a b
40% a b
16% 16% 4% 4%
Eggs
4% 4% 1% 1%
4% 4% 1% 1%
Summary of phenotypes:
a
and b
66%
a
and b
9%
a and b
9%
a and b
16%
7.8 Answer questions (a)–(e) in the preceding problem under
the assumption that the original cross was a
b/a
b
a b
/a b
.
ANS: (a) Cross: a
b/a
b a b
/a b
Gametes: a
b from one
parent, a b
from the other F
1
: a
b/a b
(b) 40% a
b, 40% a b
, 10% a
b
, 10% a b
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 21 8/14/2015 6:42:46 PM
22-WC Answers to All Questions and Problems
(c) F
2
from testcross: 40% a
b/a b, 40% a b
/a b, 10%
a
b
/a b, 10% a b/a b
(d) Repulsion linkage phase
(e) F
2
from intercross:
Sperm
40% a
+
b
a
+
b/a
+
ba
+
b/a
+
b
+
a
+
b/a ba
+
b/a b
+
a b
+
/a
+
ba b
+
/a
+
b
+
a b
+
/a ba b
+
/a b
+
a
+
b
+
/a
+
ba
+
b
+
/a
+
b
+
a
+
b
+
/a ba
+
b
+
/a b
+
a b/a
+
ba b/a
+
b
+
a b/a ba b/a b
+
10% a b
10% a
+
b
+
10% a b40% a
+
b
10% a
+
b
+
16% 16% 4% 4%
40% a b
+
40% a b
+
16% 16% 4% 4%
Eggs
4% 4% 1% 1%
4% 4% 1% 1%
Summary of phenotypes:
a
and b
51%
a
and b
24%
a and b
24%
a and b
1%
7.9 If the recombination frequency in the previous two
problems were 40 percent instead of 20 percent, what
change would occur in the proportions of gametes and
testcross progeny?
ANS: Coupling heterozygotes a
b
/a b would produce the
following gametes: 30% a
b
, 30% a b, 20% a
b, 20%
a b
; repulsion heterozygotes a
b/a b
would produce the
following gametes: 30% a
b, 30% a b
, 20% a
b
, 20%
a b. In each case, the frequencies of the testcross progeny
would correspond to the frequencies of the gametes.
7.10 A homozygous variety of maize with red leaves and nor-
mal seeds was crossed with another homozygous variety
with green leaves and tassel seeds. The hybrids were then
backcrossed to the green, tassel-seeded variety, and the
following offspring were obtained: red, normal 124; red,
tassel 126; green, normal 125; green, tassel 123. Are the
genes for plant color and seed type linked? Explain.
ANS: No. The leaf color and tassel seed traits assort
independently.
7.11 A phenotypically wild-type female fruit y that was
heterozygous for genes controlling body color and
wing length was crossed to a homozygous mutant male
with black body (allele b) and vestigial wings (allele
vg). The cross produced the following progeny: gray
body, normal wings 126; gray body, vestigial wings 24;
black body, normal wings 26; black body, vestigial
wings 124. Do these data indicate linkage between the
genes for body color and wing length? What is the
frequency of recombination? Diagram the cross,
showing the arrangement of the genetic markers on
the chromosomes.
ANS: Yes. Recombination frequency (24 26)/(126 24
26 124) 0.167. Cross:
b vg
female
×
male
b
+
vg
+
126 124 24 26
b
vg
b
+
vg
+
b vg
b
+
vg
b vg
b vg
+
b vg
b vg
b vg
b vg
7.12 Another phenotypically wild-type female fruit y het-
erozygous for the two genes mentioned in the previous
problem was crossed to a homozygous black, vestigial
male. The cross produced the following progeny: gray
body, normal wings 23; gray body, vestigial wings 127;
black body, normal wings 124; black body, vestigial wings
26. Do these data indicate linkage? What is the frequency
of recombination? Diagram the cross, showing the
arrangement of the genetic markers on the
chromosomes.
ANS: Yes. Recombination frequency (23 26)/(23 127
124 26) 0.163. Cross:
b
+
vg
female male
b
vg
+
23 26 127 124
b
vg
b
+
vg
+
b vg
b
+
vg
b vg
b vg
+
b vg
b vg
b vg
b vg
×
7.13 In rabbits, the dominant allele C is required for colored
fur; the recessive allele c makes the fur colorless (albino).
In the presence of at least one C allele, another gene
determines whether the fur is black (B, dominant) or
brown (b, recessive). A homozygous strain of brown rab-
bits was crossed with a homozygous strain of albinos.
The F
1
were then crossed to homozygous double reces-
sive rabbits, yielding the following results: black 34;
brown 66; albino 100. Are the genes b and c linked? What
is the frequency of recombination? Diagram the crosses,
showing the arrangement of the genetic markers on the
chromosomes.
ANS: Yes. Recombination frequency is estimated by the fre-
quency of black offspring among the colored offspring:
34/(66 34) 0.34. Cross:
C b c B
C
bc
B
C b
c b
c
Bc
b
C b
c bc bc
bc
b
c B c b C B
brown
albino albino
black
66 100 34
×
×
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 22 8/14/2015 6:42:48 PM
Answers to All Questions and Problems WC-23
7.14 In tomatoes, tall vine (D) is dominant over dwarf (d),
and spherical fruit shape (P) is dominant over pear
shape (p). The genes for vine height and fruit shape are
linked with 20 percent recombination between them.
One tall plant (I) with spherical fruit was crossed with
a dwarf, pear-fruited plant. The cross produced the
following results: tall, spherical 81; dwarf, pear 79;
tall, pear 22; dwarf spherical 17. Another tall plant
with spherical fruit (II) was crossed with the dwarf,
pear-fruited plant, and the following results were
obtained: tall, pear 21; dwarf, spherical 18; tall, spheri-
cal 5; dwarf, pear 4. Diagram these two crosses, show-
ing the genetic markers on the chromosomes. If the
two tall plants with spherical fruit were crossed with
each other, that is, I II, what phenotypic classes
would you expect from the cross, and in what
proportions?
ANS: Plant I has the genotype D P/d p, and when crossed to a
d p/d p plant produces four classes of progeny:
D P d p D p d P
d p d p d p d p
81 79 22 17
Plant II has the genotype D p/d P, and when crossed to a
d p/d p plant produces four classes of progeny:
D p d P D P d p
d p d p d p d p
21 18 5 4
If the two plants are crossed (D P/d p D p/d P), the
phenotypes of the offspring can be predicted from the
following table.
Gametes from plant I
Gametes
from
plant II
D p
D p/D P
0.16
d p/d p
0.04
D P/d p
0.04
d P/d p
0.16
D p/d p
0.16
d p/d P
0.01
D P/d P
0.01
d P/d P
0.04
D p/d P
0.04
d p/D p
0.01
D P/D p
0.01
d P/D p
0.04
D p/D p
0.04
D P/D P
0.04
d p/D P
0.04
d P/D P
0.16
D p
D P
0.40
0.40
0.40
0.40 0.10
0.10
0.10
0.10
D pd
pd
P
d P
d p
Summary of phenotypes:
Tall, spherical 0.54
Tall, pear
0.21
Dwarf, spherical
0.21
Dwarf, pear
0.04
7.15 In Drosophila, the genes sr (stripe thorax) and e (ebony
body) are located at 62 and 70 cM, respectively, from
the left end of chromosome 3. A striped female
homozygous for e
was mated with an ebony male
homozygous for sr
. All the offspring were phenotypi-
cally wild-type (gray body and unstriped).
(a) What kind of gametes will be produced by the F
1
females, and in what proportions?
(b) What kind of gametes will be produced by the F
1
males, and in what proportions?
(c) If the F
1
females are mated with striped, ebony
males, what offspring are expected, and in what
proportions?
(d) If the F
1
males and females are intercrossed, what off-
spring would you expect from this intercross, and in
what proportions?
ANS: (a) The F
1
females, which are sr e
/sr
e, produce four
types of gametes: 46% sr e
, 46% sr
e, 4% sr e, 4% sr
e
.
(b) The F
1
males, which have the same genotype as the F
1
females, produce two types of gametes: 50% sr e
, 50%
sr
e; remember, there is no crossing over in Drosophila
males. (c) 46% striped, gray; 46% unstriped, ebony; 4%
striped, ebony; 4% unstriped, gray. (d) The offspring
from the intercross can be obtained from the follow ing
table.
Sperm
sr e
+
0.46
0.23 0.23
0.23 0.23
0.002 0.002
0.002 0.002
sr
+
e
+
0.04
sr e
0.04
sr e
+
/sr e
+
sr e
+
/sr
+
e
0.50 0.50
sr e
+
sr
+
e
sr
+
e
0.46
sr
+
e/sr e
+
sr
+
e/sr
+
e
Eggs
sr e/sr e
+
sr e/sr
+
e
sr
+
e
+
/sr e
+
sr
+
e
+
/sr
+
e
Summary of phenotypes:
Striped, gray 0.25
Unstriped, gray 0.50
Striped, ebony 0
Unstriped, ebony 0.25
7.16 In Drosophila, genes a and b are located at positions 22.0
and 42.0 on chromosome 2, and genes c and d are
located at positions 10.0 and 25.0 on chromosome 3.
A y homozygous for the wild-type alleles of these four
genes was crossed with a y homozygous for the reces-
sive alleles, and the F
1
daughters were backcrossed to
their quadruply recessive fathers. What offspring would
you expect from this backcross, and in what
proportions?
ANS: Because the two chromosomes assort independently, the
genetic makeup of the gametes (and, therefore, of the
backcross progeny) can be obtained from the following
table.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 23 8/14/2015 6:42:49 PM
24-WC Answers to All Questions and Problems
Chromosome 3 in gametes
Chr
omo-
some
2
in
gametes
c d
a ba b c da b c
+
d
+
a
+
b
+
c d
a
+
b c d
a b
+
c da b
+
c
+
d
+
a b
+
c d
+
a b
+
c
+
d
a
+
b
+
c
+
d
+
a
+
bcd
+
a
+
b
+
c
+
d
+
a
+
b
+
c d
+
a
+
b
+
c
+
d
a
+
b c
+
d
a b c
+
da b c d
+
0.425
0.40 0.17
0.17
0.0425
0.0425
0.0425
0.0425
0.0075 0.0075
0.0075 0.0075
0.17
0.17 0.03 0.03
0.03 0.030.40
0.10
0.10
0.425 0.075 0.075
c
+
d
+
a
+
b
+
a
+
b
a b
+
c
+
dc d
+
7.17 The Drosophila genes vg (vestigial wings) and cn (cinnabar
eyes) are located at 67.0 and 57.0, respectively, on chro-
mosome 2. A female from a homozygous strain of vesti-
gial ies was crossed with a male from a homozygous
strain of cinnabar ies. The F
1
hybrids were phenotypi-
cally wild-type (long wings and dark red eyes).
(a) How many different kinds of gametes could the F
1
females produce, and in what proportions?
(b) If these females are mated with cinnabar, vestigial
males, what kinds of progeny would you expect, and in
what proportions?
ANS: (a) The F
1
females, which are cn vg
/cn
vg, produce four
types of gametes: 45% cn vg
, 45% cn
vg, 5% cn
vg
,
5% cn vg. (b) 45% cinnabar eyes, normal wings; 45%
reddish-brown eyes, vestigial wings; 5% reddish-brown
eyes, normal wings; 5% cinnabar eyes, vestigial wings.
7.18 In Drosophila, the genes st (scarlet eyes), ss (spineless bris-
tles), and e (ebony body) are located on chromosome 3,
with map positions as indicated:
st ss e
44 58 70
Each of these mutations is recessive to its wild-type allele
(st
, dark red eyes; ss
, smooth bristles; e
, gray body).
Phenotypically wild-type females with the genotype st ss
e
/st
st
ss
e were crossed with triply recessive males.
Predict the phenotypes of the progeny and the frequen-
cies with which they will occur assuming (a) no interfer-
ence and (b) complete interference.
ANS: In the following enumeration, classes 1 and 2 are paren-
tal types, classes 3 and 4 result from a single crossover
between st and ss, classes 5 and 6 result from a single
crossover between ss and e, and classes 7 and 8 result
from a double crossover, with one of the exchanges
between st and ss and the other between ss and e.
Class Phenotypes
(a) Frequency
with No
Interference
(b) Frequency
with Complete
Interference
1 Scarlet,
spineless
0.3784 0.37
2 Ebony 0.3784 0.37
3 Scarlet, ebony 0.0616 0.07
4 Spineless 0.0616 0.07
5 Scarlet,
spineless, ebony
0.0516 0.06
6 Wild-type 0.0516 0.06
7 Scarlet 0.0084 0
8 Spineless, ebony 0.0084 0
7.19 In maize, the genes Pl for purple leaves (dominant over Pl
for green leaves), sm for salmon silk (recessive to Sm for
yellow silk), and py for pigmy plant (recessive to Py for
normal-size plant) are on chromosome 6, with map posi-
tions as shown:
pl sm py
45 55 65
Hybrids from the cross Pl sm py / Pl sm py pl Sm Py / pl
Sm Py were testcrossed with pl sm py / pl sm py plants. Pre-
dict the phenotypes of the offspring and their frequen-
cies assuming (a) no interference and (b) complete
interference.
ANS: In the enumeration below, classes 1 and 2 are parental
types, classes 3 and 4 result from a single crossover
between Pl and Sm, classes 5 and 6 result from a single
crossover between Sm and Py, and classes 7 and 8 result
from a double crossover, with one of the exchanges
between Pl and Sm and the other between Sm and Py.
Class Phenotypes
(a) Frequency
with No
Interference
(b) Frequency
with Complete
Interference
1 Purple, salmon,
pigmy
0.405 0.40
2 Green, yellow,
normal
0.405 0.40
3 Purple, yellow,
normal
0.045 0.05
4 Green, salmon,
pigmy
0.045 0.05
5 Purple, salmon,
normal
0.045 0.05
6 Green, yellow,
pigmy
0.045 0.05
7 Purple, yellow,
pigmy
0.005 0
8 Green, salmon,
normal
0.005 0
7.20 In maize, the genes Tu, j2, and gl3 are located on chro-
mosome 4 at map positions 101, 106, and 112, respec-
tively. If plants homozygous for the recessive alleles of
these genes are crossed with plants homozygous for the
dominant alleles, and the F
1
plants are testcrossed to tri-
ply recessive plants, what genotypes would you expect,
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 24 8/14/2015 6:42:50 PM
Answers to All Questions and Problems WC-25
and in what proportions? Assume that interference is
complete over this map interval.
ANS: In the following enumeration, classes 1 and 2 are paren-
tal types, classes 3 and 4 result from crossing over
between Tu and j2, and classes 5 and 6 result from cross-
ing over between j2 and Gl3; only the chromosome from
the triply heterozygous F
1
plant is shown. Because inter-
ference is complete, there are no double crossover
progeny.
Class Genotype Frequency
1 tu j2 gl3 0.445
2 Tu J2 Gl3 0.445
3 tu J2 Gl3 0.025
4 Tu j2 gl3 0.025
5 tu j2 Gl3 0.030
6 Tu J2 gl3 0.030
7.21 A Drosophila geneticist made a cross between females
homozygous for three X-linked recessive mutations (y,
yellow body; ec, echinus eye shape; w, white eye color) and
wild-type males. He then mated the F
1
females to triply
mutant males and obtained the following results:
Females Males Number
/ y ec w
475
y ec w / y ec w y ec w 469
y / y ec w y
8
ec w / y ec w ec w
7
y w / y ec w y w
18
ec / y ec w ec
23
w / y ec w w
0
y ec / y ec w y ec
0
Determine the order of the three loci y, ec, and w, and
estimate the distances between them on the linkage map
of the X chromosome.
ANS: The double crossover classes, which are the two that
were not observed, establish that the gene order is
y—w—ec. Thus, the F
1
females had the genotype y w ec/
. The distance between y and w is estimated by the
frequency of recombination between these two genes:
(8 7)/1000 0.015; similarly, the distance between w
and ec is (18 23)/1000 0.041. Thus, the genetic map
for this segment of the X chromosome is y—1.5 cM—w—
4.1 cM—ec.
7.22 A Drosophila geneticist crossed females homozygous for
three X-linked mutations (y, yellow body; B, bar eye
shape; v, vermilion eye color) to wild-type males. The F
1
females, which had gray bodies and bar eyes with dark
red pigment, were then crossed to y B
v males, yielding
the following results:
Phenotype
546
244
160
50
Y
ellow, bar
vermilion
}
Y
ellow, vermilion
bar
}
Y
ellow
bar
, vermilion
}
Y
ellow, bar, vermilion
wild-type
}
Number
Determine the order of these three loci on the X chro-
mosome and estimate the distances between them.
ANS: The last two classes, consisting of yellow, bar ies and ver-
milion ies, with a total of 50 progeny, result from double
crossovers. Thus, the order of the genes is y—v—B, and
the F
1
females had the genotype y v B/ . The dis-
tance between y and v is the average number of crossovers
between them: (244 50)/1000 29.4 cM; likewise, the
distance between v and B is (160 50)/1000 21.0 cM.
Thus, the genetic map is y—29.4 cM—v—21.0 cM—B.
7.23 Female Drosophila heterozygous for three recessive
mutations e (ebony body), st (scarlet eyes), and ss (spineless
bristles) were testcrossed, and the following progeny
were obtained:
Phenotype Number
Wild-type 67
Ebony 8
Ebony, scarlet 68
Ebony, spineless 347
Ebony, scarlet, spineless 78
Scarlet 368
Scarlet, spineless 10
Spineless 54
(a) What indicates that the genes are linked?
(b) What was the genotype of the original heterozygous
females?
(c) What is the order of the genes?
(d) What is the map distance between e and st?
(e) What is the map distance between e and ss?
(f) What is the coefcient of coincidence?
(g) Diagram the crosses in this experiment.
ANS: (a) Two of the classes (the parental types) vastly outnum-
ber the other six classes (recombinant types); (b) st
/ ss e; (c) st—ss—e; (d) [(145 122) 1 (18)
2]/1000 30.3 cM; (e) (122 18)/1000 14.0 cM;
(f)(0.018)/(0.163 0.140) 0.789. (g) st / ss e
females st ss e/st ss e males → two parental classes and
six recombinant classes.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 25 8/14/2015 6:42:50 PM
26-WC Answers to All Questions and Problems
7.24 Consider a female Drosophila with the following X chro-
mosome genotype:
w dor
w
dor
The recessive alleles w and dor cause mutant eye colors
(white and deep orange, respectively). However, w is epi-
static over dor; that is, the genotypes w dor / Y and w
dor/ w dor have white eyes. If there is 40 percent recom-
bination between w and dor, what proportion of the sons
from this heterozygous female will show a mutant phe-
notype? What proportion will have either red or deep
orange eyes?
ANS: The female will produce four kinds of gametes: 30% w ,
30% dor, 20% w dor, and 20% ; thus, 80% of the
progeny will be mutant (either white or deep orange)
and 50% will be pigmented (either red or deep orange).
7.25 In Drosophila, the X-linked recessive mutations prune (pn)
and garnet (g) recombine with a frequency of 0.4. Both of
these mutations cause the eyes to be brown instead of
dark red. Females homozygous for the pn mutation were
crossed to males hemizygous for the g mutation, and the
F
1
daughters, all with dark red eyes, were crossed with
their brown-eyed brothers. Predict the frequency of sons
from this last cross that will have dark red eyes.
ANS: The F
1
females are genotypically pn / g. Among their
sons, 40 percent will be recombinant for the two X-linked
genes, and half of the recombinants will have the wild-
type alleles of these genes. Thus, the frequency of sons
with dark red eyes will be 1/2 40% 20%.
7.26 Assume that in Drosophila there are three genes x, y, and
z, with each mutant allele recessive to the wild-type
allele. A cross between females heterozygous for these
three loci and wild-type males yielded the following
progeny:
Females
1010
Males
39
z
430
y z
32
x
27
x y
441
x y z 31
Total: 2010
Using these data, construct a linkage map of the three
genes and calculate the coefcient of coincidence.
ANS: Ignore the female progeny and base the map on the male
progeny. The parental types are z and x y . The
two missing classes ( y and x z) must represent
double crossovers; thus, the gene order is y—x—z. The
distance between y and x is (32 27)/1000 5.9 cM and
that between x and z is (31 39)/1000 7.0 cM. Thus,
the map is y—5.9 cM—x—7.0 cM—z. The coefcient of
coincidence is zero.
7.27 In the nematode Caenorhabditis elegans, the linked genes
dpy (dumpy body) and unc (uncoordinated behavior) recom-
bine with a frequency P. If a repulsion heterozygote car-
rying recessive mutations in these genes is self-fertilized,
what fraction of the offspring will be both dumpy and
uncoordinated?
ANS: (P/2)
2
7.28 In the following testcross, genes a and b are 20 cM apart,
and genes b and c are 10 cM apart: a c / b a b
c / abc. If the coefcient of coincidence is 0.5 over this
interval on the linkage map, how many triply homozy-
gous recessive individuals are expected among 1000
progeny?
ANS: 5.
7.29 Drosophila females heterozygous for three recessive
mutations, a, b, and c, were crossed to males homozygous
for all three mutations. The cross yielded the following
results:
Phenotype Number
75
c
348
b c
96
a
110
a b
306
a b c 65
Construct a linkage map showing the correct order of
these genes and estimate the distances between them.
ANS: From the parental classes, c and a b , the heterozy-
gous females must have had the genotype c/a b .
The missing classes, b and a c, which would rep-
resent double crossovers, establish that the gene order
is b—a—c. The distance between b and a is (96 110)/
1000 20.6 cM and that between a and c is (65
75)/1000 14.0 cM. Thus, the genetic map is b—20.6
cM—a—14.0 cM—c.
7.30 A Drosophila second chromosome that carried a recessive
lethal mutation, l(2)g14, was maintained in a stock with a
balancer chromosome marked with a dominant muta-
tion for curly wings. This latter mutation, denoted Cy, is
also associated with a recessive lethal effect—but this
effect is different from that of l(2)g14. Thus, l(2)g14 /Cy
ies survive, and they have curly wings. Flies without the
Cy mutation have straight wings. A researcher crossed
l(2)g14 / Cy females to males that carried second chromo-
somes with different deletions (all homozygous lethal)
balanced over the Cy chromosome (genotype Df /Cy).
Each cross was scored for the presence or absence of
progeny with straight wings.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 26 8/14/2015 6:42:50 PM
Answers to All Questions and Problems WC-27
Cross
1
1n
o
2
3
4
5
6
7
8
9
10
11
12
Location of deletion Non-Curly
progeny
2n
o
3 yes
4 yes
5no
In which band is the lethal mutation l(2)g14 located?
ANS: The lethal mutation resides in band 7.
7.31 The following pedigree, described in 1937 by C. L.
Birch, shows the inheritance of X-linked color blindness
and hemophilia in a family. What is the genotype of II-2?
Do any of her children provide evidence for recombina-
tion between the genes for color blindness and
hemophilia?
Key to phenotypes:
Normal
Hemophilic
Color blind
123
I
12
II
III
1 23
ANS: II-1 has the genotype C h/c H, that is, she is a repulsion
heterozygote for the alleles for color blindness (c) and
hemophilia (h). None of her children are recombinant
for these alleles.
7.32 The following pedigree, described in 1938 by B. Rath,
shows the inheritance of X-linked color blindness and
hemophilia in a family. What are the possible genotypes
of II-1? For each possible genotype, evaluate the chil-
dren of II-1 for evidence of recombination between the
color blindness and hemophilia genes.
I
12
12
II
III
12 3 4
Key to phenotypes:
Normal
Hemophilic
Color vision uncertain
Color blind and hemophilic
Color blind
ANS: II-1 is either (a) C h/c H or (b) c h/C H. Her four sons
have the genotypes c h (1), C h (2), c H (3), and C H (4). If
II-1 has the genotype C h/c H, sons 1 and 4 are recombi-
nant and sons 2 and 3 are nonrecombinant. If II-1 has
the genotype c h/C H, sons 2 and 3 are recombinant and
sons 1 and 4 are nonrecombinant. Either way, the fre-
quency of recombination is 0.5.
7.33 A normal woman with a color-blind father married a
normal man, and their rst child, a boy, had hemophilia.
Both color blindness and hemophilia are due to X-linked
recessive mutations, and the relevant genes are separated
by 10 cM. This couple plans to have a second child. What
is the probability that it will have hemophilia? Color
blindness? Both hemophilia and color blindness? Nei-
ther hemophilia nor color blindness?
ANS: The woman is a repulsion heterozygote for the alleles
for color blindness and hemophilia—that is, she is C h/c
H. If the woman has a boy, the chance that he will have
hemophilia is 0.5 and the chance that he will have color
blindness is 0.5. If we specify that the boy have only one
of these two conditions, then the chance that he will
have color blindness is 0.45. The reason is that the boy
will inherit a nonrecombinant X chromosome with a
probability of 0.9, and half the nonrecombinant X chro-
mosomes will carry the mutant allele for color blindness
and the other half will carry the mutant allele for hemo-
philia. The chance that the boy will have both conditions
is 0.05, and the chance that he will have neither condi-
tion is 0.05. The reason is that the boy will inherit a
recombinant X chromosome with a probability of 0.1,
and half the recombinant X chromosomes will carry
both mutant alleles and the other half will carry neither
mutant allele.
7.34 Two strains of maize, M1 and M2, are homozygous for
four recessive mutations, a, b, c, and d, on one of the
large chromosomes in the genome. Strain W1 is
homozygous for the dominant alleles of these muta-
tions. Hybrids produced by crossing M1 and W1 yield
many different classes of recombinants, whereas
hybrids produced by crossing M2 and W1 do not yield
any recombinants at all. What is the difference between
M1 and M2?
ANS: M2 carries an inversion that suppresses recombination in
the chromosome.
7.35 A Drosophila geneticist has identied a strain of ies with
a large inversion in the left arm of chromosome 3. This
inversion includes two mutations, e (ebony body) and cd
(cardinal eyes), and is anked by two other mutations, sr
(stripe thorax) on the right and ro (rough eyes) on the left.
The geneticist wishes to replace the e and cd mutations
inside the inversion with their wild-type alleles; he plans
to accomplish this by recombining the multiply mutant,
inverted chromosome with a wild-type, inversion-free
chromosome. What event is the geneticist counting on
to achieve his objective? Explain.
ANS: A two-strand double crossover within the inversion;
the exchange points of the double crossover must lie
between the genetic markers and the inversion
breakpoints.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 27 8/14/2015 6:42:51 PM

28-WC Answers to All Questions and Problems
Chapter 8
8.1 By what criteria are viruses living? nonliving?
ANS: Viruses reproduce and transmit their genes to progeny
viruses. They utilize energy provided by host cells and
respond to environmental and cellular signals like other
living organisms. However, viruses are obligate parasites;
they can reproduce only in appropriate host cells.
8.2 How do bacteriophages differ from other viruses?
ANS: Bacteriophages reproduce in bacteria; other viruses
reproduce in protists, plants, and animals.
8.3 In what ways do the life cycles of bacteriophages T4 and
l differ? In what aspects are they the same?
ANS: Bacteriophage T4 is a virulent phage. When it infects a
host cell, it reproduces and kills the host cell in the pro-
cess. Bacteriophage lambda can reproduce and kill the
host bacterium—the lytic response—just like phage T4,
or it can insert its chromosome into the chromosome of
the host and remain there in a dormant state—the lyso-
genic response.
8.4 How does the structure of the l prophage differ from the
structure of the l chromosome packaged in the l head?
ANS: The mature (packaged) lambda chromosome and the
lambda prophage are circular permutations of one
another (see Figure 8.5).
8.5 In what way does the integration of the l chromosome
into the host chromosome during a lysogenic infection
differ from crossing over between homologous
chromosomes?
ANS: The insertion of the phage l chromosome into the host
chromosome is a site-specic recombination process
catalyzed by an enzyme that recognizes specic sequences
in the l and E. coli chromosomes. Crossing over between
homologous chromosomes is not sequence specic. It
can occur at many sites along the two chromosomes.
8.6 Geneticists have used mutations that cause altered pheno-
types such as white eyes in Drosophila, white owers and
wrinkled seeds in peas, and altered coat color in rabbits to
determine the locations of genes on the chromosomes of
these eukaryotes. What kinds of mutant phenotypes have
been used map genes in bacteria?
ANS: Three main types of bacterial mutants have been used to
map genes in bacteria; these include mutants unable to
utilize specic sugars as energy sources (such as lactose),
mutants unable to synthesize essential metabolites (these
are called auxotrophs), and mutants resistant to drugs
and antibiotics. Wild-type bacteria can use almost any
sugar as an energy source, can grow on minimal media,
and are killed by antibiotics, whereas mutants in genes
controlling these processes result in different growth
characteristics. These growth phenotypes can be used to
map genes in bacteria.
8.7 You have identied three mutations—a, b, and c—in
Streptococcus pneumoniae. All three are recessive to their
wild-type alleles a
, b
, and c
. You prepare DNA from a
wild-type donor strain and use it to transform a strain
with genotype a b c. You observe a
b
transformants and
a
c
transformants, but no b
c
transformants. Are these
mutations closely linked? If so, what is their order on the
Streptococcus chromosome?
ANS: The a, b, and c mutations are closely linked and in the
order b—a—c on the chromosome.
8.8 A nutritionally defective E. coli strain grows only on a
medium containing thymine, whereas another nutrition-
ally defective strain grows only on a medium containing
leucine. When these two strains were grown together, a
few progeny were able to grow on a minimal medium
with neither thymine nor leucine. How can this result be
explained?
ANS: There are two possible explanations. One possibility is
that a spontaneous mutation caused reversion of either
auxotrophic strain to the prototrophic condition. Because
this requires only one mutation in one cell, this is a pos-
sibility, although rare. Another, more likely, possibility is
that conjugation occurred between the E. coli parental
auxotrophic strains. During conjugation, genes from the
parental strains recombined. Because each parent had a
wild-type gene copy for either thymine or leucine, recom-
binant progeny containing the wild-type copy of each
gene would be able to synthesize both nutrients and grow
on minimal medium.
8.9 Assume that you have just demonstrated genetic recom-
bination (e.g., when a strain of genotype a b
is present
with a strain of genotype a
b, some recombinant geno-
types, a
b
and a b, are formed) in a previously unstudied
species of bacteria. How would you determine whether
the observed recombination resulted from transforma-
tion, conjugation, or transduction?
ANS: Perform two experiments: (1) determine whether the
process is sensitive to DNase and (2) determine whether
cell contact is required for the process to take place. The
cell contact requirement can be tested by a U-tube
experiment (see Figure 8.9). If the process is sensitive to
DNase, it is similar to transformation. If cell contact is
required, it is similar to conjugation. If it is neither sensi-
tive to DNase nor requires cell contact, it is similar to
transduction.
8.10 (a) What are the genotypic differences between F
-
cells,
F
cells, and Hfr cells? (b) What are the phenotypic dif-
ferences? (c) By what mechanism are F
cells converted
into F
cells? F
cells to Hfr cells? Hfr cells to F
cells?
ANS: (a) F
cells, no F factor present; F
cells, autonomous F
factor; Hfr cells, integrated F factor (see Figure 8.14).
(b) F
and Hfr cells have F pili; F
cells do not. (c) F
cells are converted into F
cells by the conjugative trans-
fer of F factors from F
cells. Hfr cells are formed when
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 28 8/14/2015 6:42:51 PM

Answers to All Questions and Problems WC-29
F factors in F
cells become integrated into the chromo-
somes of these cells. Hfr cells become F
cells when the
integrated F factors exit the chromosome and become
autonomous (self-replicating) genetic elements.
8.11 (a) Of what use are F′ factors in genetic analysis? (b) How
are F′ factors formed? (c) By what mechanism does sex-
duction occur?
ANS: (a) F′ factors are useful for genetic analyses where two
copies of a gene must be present in the same cell, for
example, in determining dominance relationships. (b) F′
factors are formed by abnormal excision of F factors
from Hfr chromosomes (see Figure 8.21). (c) By the con-
jugative transfer of an F′ factor from a donor cell to a
recipient (F
) cell.
8.12 What are the basic differences between generalized
transduction and specialized transduction?
ANS: Generalized transduction: (1) transducing particles often
contain only host DNA; (2) transducing particles may
carry any segment of the host chromosome. Thus, all
host genes are transduced. Specialized transduction:
(1) transducing particles carry a recombinant chromo-
some, which contains both phage DNA and host DNA;
(2) only host genes that are adjacent to the prophage
integration site are transduced.
8.13 What roles do IS elements play in the integration of F
factors?
ANS: IS elements (or insertion sequences) are short (800–
1400 nucleotide pairs) DNA sequences that are trans-
posable—that is, capable of moving from one position
in a chromosome to another position or from one
chromosome to another chromosome. IS elements
mediate recombination between nonhomologous
DNA molecules—for example, between F factors and
bacterial chromosomes.
8.14 How can bacterial genes be mapped by interrupted mat-
ing experiments?
ANS: By interrupting conjugation at various times after the
donor and recipient cells are mixed (using a blender or
other form of agitation), one can determine the length of
time required to transfer a given genetic marker from an
Hfr cell to an F
.
8.15 What does the term cotransduction mean? How can
cotransduction frequencies be used to map genetic
markers?
ANS: Cotransduction refers to the simultaneous transduction
of two different genetic markers to a single recipient
cell. Since bacteriophage particles can package only
1/100 to 1/50 of the total bacterial chromosome, only
markers that are relatively closely linked can be cotrans-
duced. The frequency of cotransduction of any two
markers will be an inverse function of the distance
between them on the chromosome. As such, this
frequency can be used as an estimate of the linkage dis-
tance. Specic cotransduction-linkage functions must be
prepared for each phage–host system studied.
8.16 In E. coli, the ability to utilize lactose as a carbon source
requires the presence of the enzymes b-galactosidase and
b-galactoside permease. These enzymes are encoded by
two closely linked genes, lacZ and lacY, respectively.
Another gene, proC, controls, in part, the ability of E. coli
cells to synthesize the amino acid proline. The alleles str
r
and str
s
, respectively, control resistance and sensitivity to
streptomycin. Hfr H is known to transfer the two lac
genes, proC, and str, in that order, during conjugation. A
cross was made between Hfr H of genotype lacZ
lacY
proC
str
s
and an F
strain of genotype lacZ
lacY
proC
str
r
. After about 2 hours, the mixture was diluted and
plated out on medium containing streptomycin but no
proline. When the resulting proC
str
r
recombinant colo-
nies were checked for their ability to grow on medium
containing lactose as the sole carbon source, very few of
them were capable of fermenting lactose. When the
reciprocal cross (Hfr H lacZ
lacY
proC
str
s
X F
lacZ
lacY
proC
str
r
) was done, many of the proC
str
r
recom-
binants were able to grow on medium containing lactose
as the sole carbon source. What is the order of the lacZ
and lacY genes relative to proC?
ANS: lacY—lacZ—proC.
8.17 An F
strain, marked at 10 loci, gives rise spontaneously
to Hfr progeny whenever the F factor becomes incorpo-
rated into the chromosome of the F
strain. The F factor
can integrate into the circular chromosome at many
points, so that the resulting Hfr strains transfer the
genetic markers in different orders. For any Hfr strain,
the order of markers entering a recipient cell can be
determined by interrupted mating experiments. From
the following data for several Hfr strains derived from
the same F
, determine the order of markers in the F
strain.
Hfr Strain Markers Donated in order
1 —Z–H–E–R→
2 —O–K–S–R→
3 —K–O–W–I→
4 —Z–T–I–W→
5 —H–Z–T–I→
ANS:
Z
K
HT
E
I
R
W
SO
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 29 8/14/2015 6:42:51 PM
30-WC Answers to All Questions and Problems
8.18 The data in the following table were obtained from
three-point transduction tests made to determine the
order of mutant sites in the A gene encoding the a sub-
unit of tryptophan synthetase in E. coli. Anth is a linked,
unselected marker. In each cross, trp
recombinants were
selected and then scored for the anth marker (anth
or
anth
-
). What is the linear order of anth and the three
mutant alleles of the A gene indicated by the data in
the table?
Cross
Donor
Markers
Recipient
Markers
anth Allele in trp
Recombinants % anth
1
anth
—
A34
anth
—
A223
72 anth
: 332 anth
18
2
anth
—
A46
anth
—
A223
196 anth
: 180
anth
52
3
anth
—
A223
anth
—
A34
380 anth
: 379
anth
50
4
anth
—
A223
anth
—
A46
60 anth
: 280 anth
20
ANS: anth—A34—A223—A46.
8.19 Bacteriophage P1 mediates generalized transduction
in E. coli. A P1 transducing lysate was prepared by
growing P1 phage on pur
pro
-
his
-
bacteria. Genes
pur, pro, and his encode enzymes required for the syn-
thesis of purines, proline, and histidine, respectively.
The phage and transducing particles in this lysate
were then allowed to infect pur
pro
his
cells. After
incubating the infected bacteria for a period of time
sufcient to allow transduction to occur, they were
plated on minimal medium supplemented with proline
and histidine, but no purines to select for pur
trans-
ductants. The pur
colonies were then transferred to
minimal medium with and without proline and with
and without histidine to determine the frequencies of
each of the outside markers. Given the following
results, what is the order of the three genes on the
E. coli chromosome?
Genotype Number Observed
pro
his
100
pro
his
22
pro
his
-
150
pro
-
his
-
1
ANS: pro—pur—his.
8.20 Two additional mutations in the trp A gene of E. coli, trp
A58 and trp A487, were ordered relative to trp A223 and
the outside marker anth by three-factor transduction
crosses as described in Problem 8.18. The results of these
crosses are summarized in the following table. What is
the linear order of anth and the three mutant sites in the
trp A gene?
Cross
Donor
Markers
Recipient
Markers
anth Allele in trp
Recombinants
%
anth
1
anth
—
A487
anth
—
A223
72 anth
:
332 anth
82
2
anth
—
A58
anth
—
A223
196 anth
:
180 anth
48
3
anth
—
A223
anth
—
A487
380 anth
:
379 anth
50
4
anth
—
A223
anth
—
A58
60 anth
:
280 anth
80
ANS: anth—A487—A223—A58.
8.21 You have identied a mutant E. coli strain that cannot
synthesize histidine (His
). To determine the location
of the his
mutation on the E. coli chromosome, you
perform interrupted mating experiments with ve dif-
ferent Hfr strains. The following chart shows the time
of entry (minutes, in parentheses) of the wild-type
alleles of the rst ve markers (mutant genes) into the
His
strain.
Hfr A -------- his (1) man (9) gal (28) lac (37) thr (45)
Hfr B -------- man (15) his (23) cys (38) ser (42) arg (49)
Hfr C -------- thr (3) lac (11) gal (20) man (39) his (47)
Hfr D -------- cys (3) his (18) man (26) gal (45) lac (54)
Hfr E -------- thr (6) rha (18) arg (36) ser (43) cys (47)
On the map below of the circular E. coli chromosome,
indicate (1) the relative location of each gene relative
to thr (located at 0/100 Min), (2) the position where
the sex factor is integrated in each of the ve Hfr’s,
and (3) the direction of chromosome transfer for each
Hfr (indicate direction with an arrow or arrowhead).
thr
lac
100/0
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 30 8/14/2015 6:42:52 PM
Answers to All Questions and Problems WC-31
ANS:
thr
lac
gal
man
his
cys
ser
ar
g
rha
Min
E
C
B
A
D
0/100
8
17
36
44
59
63
70
88
8.22 Mutations nrd 11 (gene nrd B, encoding the beta subunit
of the enzyme ribonucleotide reductase), am M69 (gene
63, encoding a protein that aids tail-ber attachment),
and nd 28 (gene denA, encoding the enzyme endonucle-
ase II) are known to be located between gene 31 and
gene 32 on the bacteriophage T4 chromosome. Muta-
tions am N54 and am A453 are located in genes 31 and
32, respectively. Given the three-factor cross data in the
following table, what is the linear order of the ve
mutant sites?
Three-Factor Cross Data
Cross % Recombination
a
1. am A453—am M69 nrd 11
2.6
2. am A453—am M69 nrd 11
4.2
3. am A453—am M69 nd 28
2.5
4. am A453—nd 28 am M69
3.5
5. am A453—nrd 11 nd 28
2.9
6. am A453—nd 28 nrd 11
2.1
7. am N54—am M69 nrd 11
3.5
8. am N54—nrd 11 am M69
1.9
9. am N54—nd 28 am M69
1.7
10. am N54—am M69 nd 28
2.7
11. am N54—nd 28 nrd 11
2.9
12. am N54—nrd 11 nd 28
1.9
a
All recombination frequencies are given as
2
(wild type progeny)
100.
total progeny
ANS: amA453—nrd11—nd28—amM69—amN54.
Chapter 9
9.1 (a) How did the transformation experiments of Grifth
differ from those of Avery and his associates? (b) What was
the signicant contribution of each? (c) Why was Grifth’s
work not evidence for DNA as the genetic material,
whereas the experiments of Avery and coworkers provided
direct proof that DNA carried the genetic information?
ANS: (a) Grifth’s in vivo experiments demonstrated the occur-
rence of transformation in pneumococcus. They pro-
vided no indication as to the molecular basis of the
transformation phenomenon. Avery and colleagues car-
ried out in vitro experiments, employing biochemical
analyses to demonstrate that transformation was medi-
ated by DNA. (b) Grifth showed that a transforming
substance existed; Avery et al. dened it as DNA. (c)
Grifth’s experiments did not include any attempt to
characterize the substance responsible for transforma-
tion. Avery et al. isolated DNA in “pure” form and dem-
onstrated that it could mediate transformation.
9.2 A cell-free extract is prepared from Type IIIS pneumo-
coccal cells. What effect will treatment of this extract
with (a) protease, (b) RNase, and (c) DNase have on its
subsequent capacity to transform recipient Type IIR cells
to Type IIIS? Why?
ANS: (a) No effect; (b) no effect; (c) DNase will destroy the
capacity of the extract to transform type IIR cells to Type
IIIS by degrading the DNA in the extract. Protease and
RNase will degrade the proteins and RNA, respectively,
in the extract. They will have no effect, since the proteins
and RNA are not involved in transformation.
9.3 How could it be demonstrated that the mixing of heat-
killed Type III pneumococcus with live Type II resulted in
a transfer of genetic material from Type III to Type II
rather than a restoration of viability to Type III by Type II?
ANS: Puried DNA from Type III cells was shown to be
sufcient to transform Type II cells. This occurred in the
absence of any dead Type III cells.
9.4 What is the macromolecular composition of a bacterial
virus or bacteriophage such as phage T2?
ANS: About 1/2 protein, 1/2 DNA. A single long molecule of
DNA is enclosed within a complex “coat” composed of
many proteins.
9.5 (a) What was the objective of the experiment carried out
by Hershey and Chase? (b) How was the objective
accomplished? (c) What is the signicance of this
experiment?
ANS: (a) The objective was to determine whether the genetic
material was DNA or protein. (b) By labeling phospho-
rus, a constituent of DNA, and sulfur, a constituent of
protein, in a virus, it was possible to demonstrate that
only the labeled phosphorus was introduced into the
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 31 8/14/2015 6:42:52 PM

32-WC Answers to All Questions and Problems
host cell during the viral reproductive cycle. The DNA
was enough to produce new phages. (c) Therefore DNA,
not protein, is the genetic material.
9.6 How did the reconstitution experiment of Fraenkel–
Conrat and colleagues show that the genetic information
of tobacco mosaic virus (TMV) is stored in its RNA
rather than its protein?
ANS: When tobacco leaves were infected with reconstituted
virus particles containing RNA from type A viruses and
protein from type B viruses, the progeny viruses were
type A, showing that RNA, not protein, carries the
genetic information in TMV.
9.7 (a) What background material did Watson and Crick
have available for developing a model of DNA? (b) What
was their contribution to building the model?
ANS: (a) The ladder-like pattern was known from X-ray dif-
fraction studies. Chemical analyses had shown that a 1:1
relationship existed between the organic bases adenine
and thymine and between cytosine and guanine. Physical
data concerning the length of each spiral and the stack-
ing of bases were also available. (b) Watson and Crick
developed the model of a double helix, with the rigid
strands of sugar and phosphorus forming spirals around
an axis, and hydrogen bonds connecting the complemen-
tary bases in nucleotide pairs.
9.8 (a) Why did Watson and Crick choose a double helix for
their model of DNA structure? (b) Why were hydrogen
bonds placed in the model to connect the bases?
ANS: (a) A multistranded, spiral structure was suggested by the
X-ray diffraction patterns. A double-stranded helix with
specic base-pairing nicely ts the 1:1 stoichiometry
observed for A:T and G:C in DNA. (b) Use of the known
hydrogen-bonding potential of the bases provided a
means of holding the two complementary strands in a
stable conguration in such a double helix.
9.9 (a) If a virus particle contained double-stranded DNA
with 200,000 base pairs, how many nucleotides would be
present? (b) How many complete spirals would occur on
each strand? (c) How many atoms of phosphorus would
be present? (d) What would be the length of the DNA
conguration in the virus?
ANS: (a) 400,000; (b) 20,000; (c) 400,000; (d) 68,000 nm.
9.10 What are the differences between DNA and RNA?
ANS: DNA has one atom less of oxygen than RNA in the sugar
part of the molecule; the sugar in DNA is 2-deoxyribose,
whereas the sugar in RNA is ribose. In DNA, thymine
replaces the uracil that is present in RNA. (In certain
bacteriophages, DNA-containing uracil is present.)
DNA is most frequently double-stranded, but bacterio-
phages such as ΦX174 contain single-stranded DNA.
RNA is most frequently single-stranded. Some viruses,
such as the Reoviruses, however, contain double-stranded
RNA chromosomes.
9.11 RNA was extracted from TMV (tobacco mosaic virus) par-
ticles and found to contain 20 percent cytosine (20 percent
of the bases were cytosine). With this information, is it
possible to predict what percentage of the bases in TMV
are adenine? If so, what percentage? If not, why not?
ANS: No. TMV RNA is single-stranded. Thus, the base-pair
stoichiometry of DNA does not apply.
9.12 DNA was extracted from cells of Staphylococcus afermen-
tans and analyzed for base composition. It was found that
37 percent of the bases are cytosine. With this informa-
tion, is it possible to predict what percentage of the bases
are adenine? If so, what percentage? If not, why not?
ANS: Yes. Because DNA in bacteria is double-stranded, the 1:1
base-pair stoichiometry applies. Therefore, if 37% of the
bases are cytosine, then 37% are guanine. This means
that the remaining 26% of the bases are adenine and thy-
mine. Thus, 26%/2 13% of the bases are adenine.
9.13 If one strand of DNA in the Watson–Crick double helix
has a base sequence of 5-GTCATGAC-3, what is the
base sequence of the complementary strand?
ANS: 3′-C A G T A C T G-5′
9.14 Indicate whether each of the following statements about
the structure of DNA is true or false. (Each letter is used
to refer to the concentration of that base in DNA.)
(a) A T G C
(b) A G; C T
(c) A/T C/G
(d) T/A C/G
(e) A G C T
(f) G/C 1
(g) A T within each single strand.
(h) Hydrogen bonding provides stability to the double
helix in aqueous cytoplasms.
(i) Hydrophobic bonding provides stability to the double
helix in aqueous cytoplasms.
(j) When separated, the two strands of a double helix are
identical.
(k) Once the base sequence of one strand of a DNA dou-
ble helix is known, the base sequence of the second
strand can be deduced.
(l) The structure of a DNA double helix is invariant. (m)
Each nucleotide pair contains two phosphate groups,
two deoxyribose molecules, and two bases.
ANS: (a) False (b) False (c) True (d) True (e) True
(f) True (g) False (h) True (i) True (j) False
(k) Frue (l) False (m) True
9.15 The nucleic acids from various viruses were extracted
and examined to determine their base composition.
Given the following results, what can you hypothesize
about the physical nature of the nucleic acids from these
viruses?
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 32 8/14/2015 6:42:52 PM

Answers to All Questions and Problems WC-33
(a) 35% A, 35% T, 15% G, and 15% C. (b) 35% A, 15% T,
25% G, and 25% C. (c) 35% A, 30% U, 30% G, and 5% C.
ANS: (a) Double-stranded DNA; (b) single-stranded DNA;
(c) single-stranded RNA.
9.16 Compare and contrast the structures of the A, B, and Z
forms of DNA.
ANS: The B form of DNA helix is that proposed by Watson
and Crick and is the conformation that DNA takes under
physiological conditions. It is a right-handed double
helical coil with 10 bases per turn of the helix and a diam-
eter of 1.9 nm. It has a major and a minor groove. Z-DNA
is left-handed, has 12 bases per turn, a single deep groove,
and is 1.8 nm in diameter. Its sugar–phosphate backbone
takes a zigzagged path, and it is G:C rich. A-DNA is a
right-handed helix with 11 base pairs per turn. It is a
shorter, thicker double helix with a diameter of 0.23 nm
and has a narrow, deep major groove and a broad, shal-
low minor groove. A-DNA forms in vitro under high salt
concentrations or in a partially dehydrated state.
9.17 The temperature at which one-half of a double-stranded
DNA molecule has been denatured is called the melting
temperature, T
m
. Why does T
m
depend directly on the
GC content of the DNA?
ANS: The value of T
m
increases with the GC content because
GC base pairs, connected by three hydrogen bonds, are
stronger than AT base pairs connected by two hydrogen
bonds.
9.18 A diploid rye plant, Secale cereale, has 2n 14 chromo-
somes and approximately 1.6 10
10
bp of DNA. How
much DNA is in a nucleus of a rye cell at (a) mitotic
metaphase, (b) meiotic metaphase I, (c) mitotic telo-
phase, and (d) meiotic telophase II?
ANS: (a) 3.2 10
10
bp (b) 3.2 10
10
bp (c) 1.6 10
10
bp (d) 0.8
10
10
bp
9.19 The available evidence indicates that each eukaryotic
chromosome (excluding polytene chromosomes) con-
tains a single giant molecule of DNA. What different
levels of organization of this DNA molecule are appar-
ent in chromosomes of eukaryotes at various times dur-
ing the cell cycle?
ANS: DNA during interphase is not yet organized into indi-
vidual chromosomes but consists of a series of ellipsoidal
“beads on a string” that form an 11-nm ber. Here, 146
bp of DNA is wrapped 1.65 turns around the nucleo-
some core of eight histones. However, during metaphase
of meiosis and mitosis, DNA becomes organized into
chromosomes. The 11-nm ber is folded and super-
coiled to produce a 30-nm chromatin ber, the basic
structural unit of the metaphase chromosome. A third
and nal level of packaging involves nonhistone chro-
mosomal proteins that form a scaffold to condense the
30-nm bers into tightly packaged metaphase chromo-
somes, the highest level of DNA condensation observed.
9.20 A diploid nucleus of Drosophila melanogaster contains
about 3.4 10
8
nucleotide pairs. Assume (1) that all
nuclear DNA is packaged in nucleosomes and (2) that an
average internucleosome linker size is 60 nucleotide
pairs. How many nucleosomes would be present in a dip-
loid nucleus of D. melanogaster? How many molecules of
histone H2a, H2b, H3, and H4 would be required?
ANS: In the diploid nucleus of D. melanogaster, 1.65 10
6
nucleosomes would be present; these would contain 3.3
10
6
molecules of each histone, H2a, H2b, H3, and H4.
9.21 The relationship between the melting T
m
and GC con-
tent can be expressed, in its much simplied form, by the
formula T
m
69 0.41 (% GC). (a) Calculate the melt-
ing temperature of E. coli DNA that has about 50% GC.
(b) Estimate the %GC of DNA from a human kidney
cell where T
m
85°C.
ANS: (a) 89.5°C. (b) About 39%
9.22 Experimental evidence indicates that most highly repeti-
tive DNA sequences in the chromosomes of eukaryotes
do not produce any RNA or protein products. What
does this indicate about the function of highly repetitive
DNA?
ANS: It indicates that most highly repetitive DNA sequences
do not contain structural genes specifying RNA and
polypeptide gene products.
9.23 The satellite DNAs of Drosophila virilis can be isolated,
essentially free of main-fraction DNA, by density-gradi-
ent centrifugation. If these satellite DNAs are sheared
into approximately 40-nucleotide-pair-long fragments
and are analyzed in denaturation–renaturation experi-
ments, how would you expect their hybridization kinet-
ics to compare with the renaturation kinetics observed
using similarly sheared main-fraction DNA under the
same conditions? Why?
ANS: The satellite DNA fragments would renature much
more rapidly than the main-fraction DNA fragments. In
D. virilus satellite DNAs, all three have repeating hepta-
nucleotide-pair sequences. Thus, essentially every 40
nucleotide-long (average) single-stranded fragment
from one strand will have a sequence complementary (in
part) with every single-stranded fragment from the com-
plementary strand. Many of the nucleotide-pair
sequences in main-fraction DNA will be unique
sequences (present only once in the genome).
9.24 (a) What functions do (1) centromeres and (2) telomeres
provide? (b) Do telomeres have any unique structural
features? (c) When chromosomes are broken by expo-
sure to high-energy radiation such as X rays, the result-
ing broken ends exhibit a pronounced tendency to stick
to each other and fuse. Why might this occur?
ANS: (a) (1) Centromeres function as spindle-ber attachment
sites on chromosomes; they are required for the separa-
tion of homologous chromosomes to opposite poles of
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 33 8/14/2015 6:42:52 PM

34-WC Answers to All Questions and Problems
the spindle during anaphase I of meiosis and for the
separation of sister chromatids during anaphase of mito-
sis and anaphase II of meiosis. (2) Telomeres provide at
least three important functions: (i) prevention of exonu-
cleolytic degradation of the ends of the linear DNA mol-
ecules in eukaryotic chromosomes, (ii) prevention of the
fusion of ends of DNA molecules of different chromo-
somes, and (iii) provision of a mechanism for replication
of the distal tips of linear DNA molecules in eukaryotic
chromosomes. (b) Yes. Most telomeres studied to date
contain DNA sequence repeat units (e.g., TTAGGG in
human chromosomes), and at least in some species, telo-
meres terminate with single-stranded 3′ overhangs that
form “hairpin” structures. The bases in these hairpins
exhibit unique patterns of methylation that presumably
contribute to the structure and stability of telomeres.
(c) The broken ends resulting from irradiation will not
contain telomeres; as a result, the free ends of the DNA
molecules are apparently subject to the activities of
enzymes such as exonucleases, ligases, and so on, which
modify the ends. They can regain stability by fusing to
broken ends of other DNA molecules that contain ter-
minal telomere sequences.
9.25 Are eukaryotic chromosomes metabolically most active
during prophase, metaphase, anaphase, telophase, or
interphase?
ANS: Interphase. Chromosomes are for the most part meta-
bolically inactive (exhibiting little transcription) during
the various stages of condensation in mitosis and
meiosis.
9.26 Are the scaffolds of eukaryotic chromosomes composed
of histone or nonhistone chromosomal proteins? How
has this been determined experimentally?
ANS: Nonhistone chromosomal proteins. The “scaffold”
structures of metaphase chromosomes can be observed
by light microscopy after removal of the histones by dif-
ferential extraction procedures.
9.27 (a) Which class of chromosomal proteins, histones or
nonhistones, is the more highly conserved in different
eukaryotic species? Why might this difference be
expected? (b) If one compares the histone and nonhis-
tone chromosomal proteins of chromatin isolated from
different tissues or cell types of a given eukaryotic organ-
ism, which class of proteins will exhibit the greater het-
erogeneity? Why are both classes of proteins not
expected to be equally homogeneous in chromosomes
from different tissues or cell types?
ANS: (a) Histones have been highly conserved throughout the
evolution of eukaryotes. A major function of histones is
to package DNA into nucleosomes and chromatin bers.
Since DNA is composed of the same four nucleotides
and has the same basic structure in all eukaryotes, one
might expect that the proteins that play a structural role
in packaging this DNA would be similarly conserved. (b)
The nonhistone chromosomal proteins exhibit the
greater heterogeneity in chromatin from different tis-
sues and cell types of an organism. The histone composi-
tion is largely the same in all cell types within a given
species—consistent with the role of histones in packag-
ing DNA into nucleosomes. The nonhistone chromo-
somal proteins include proteins that regulate gene
expression. Because different sets of genes are tran-
scribed in different cell types, one would expect hetero-
geneity in some of the nonhistone chromosomal proteins
of different tissues.
9.28 (a) If the haploid human genome contains 3 10
9
nucle-
otide pairs and the average molecular weight of a nucleo-
tide pair is 660, how many copies of the human genome
are present, on average, in 1 mg of human DNA?
(b) What is the weight of one copy of the human genome?
(c) If the haploid genome of the small plant Arabidopsis
thaliana contains 7.7 107 nucleotide pairs, how many
copies of the A. thaliana genome are present, on average,
in 1 mg of A. thaliana DNA? (d) What is the weight of
one copy of the A. thaliana genome? (e) Of what impor-
tance are calculations of the above type to geneticists?
ANS: (a) One microgram of human DNA will contain, on
average, 3.04 10
5
copies of the genome. Using an
average molecular weight per nucleotide pair of 660,
the molecular weight of the entire human genome is
1.98 10
12
(3 10
9
660). Thus, 1.98 10
12
g
(1 “mole” number of grams equivalent to the “molec-
ular” weight) of human DNA will contain, on average,
6.02 10
23
molecules [Avogadro’s number number of
molecules (here, copies of the genome) present in one
“mole” of a substance]. One gram will contain on aver-
age (3.04 10
11
)(6.02 10
23
/1.98 10
12
) copies of the
genome; thus, 1 mg will contain, on average, 3.04 10
5
copies of the human genome. (b) One copy of the
human genome weighs approximately (3.3 10
12
g)
(1.98 10
12
g per “mole”/6.02 10
23
molecules per
“mole”) or 3.3 10
6
mg. (c) By analogous calculations,
1 g of A. thaliana DNA contains, on average, 1.18 10
7
copies of the genome. (d) Similarly, one copy of the A.
thaliana genome weighs approximately 8.4 10
8
mg.
(e) In carrying out molecular analyses of the structures
of genomes, geneticists frequently need to know how
many copies of a genome are present, on average, in a
given quantity of DNA.
Chapter 10
10.1 DNA polymerase I of E. coli is a single polypeptide of
molecular weight 103,000.
(a) What enzymatic activities other than polymerase
activity does this polypeptide possess? (b) What are the
in vivo functions of these activities? (c) Are these activi-
ties of major importance to an E. coli cell? Why?
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 34 8/14/2015 6:42:52 PM

Answers to All Questions and Problems WC-35
ANS: (a) Both 3′ → 5′ and 5′ → 3′ exonuclease activities.
(b) The 3′ → 5′ exonuclease “proofreads” the nascent
DNA strand during its synthesis. If a mismatched base
pair occurs at the 3′-OH end of the primer, the 3′ → 5′
exonuclease removes the incorrect terminal nucleotide
before polymerization proceeds again. The 5′ → 3′ exo-
nuclease is responsible for the removal of RNA primers
during DNA replication and functions in pathways
involved in the repair of damaged DNA (see Chapter
13). (c) Yes, both exonuclease activities appear to be very
important. Without the 3′ → 5′ proofreading activity
during replication, an intolerable mutation frequency
would occur. The 5′ → 3′ exonuclease activity is essential
to the survival of the cell. Conditional mutations that
alter the 5′ → 3′ exonuclease activity of DNA polymerase
I are lethal to the cell under conditions where the exo-
nuclease is nonfunctional.
10.2 Escherichia coli cells are grown for many generations in a
medium in which the only available nitrogen is the heavy
isotope
15
N. They are then transferred to a medium con-
taining
14
N as the only source of nitrogen.
(a) What distribution of
15
N and
14
N would be expected
in the DNA molecules of cells that had grown for one
generation in the
14
N-containing medium assuming that
DNA replication was (i) conservative, (ii) semiconserva-
tive, or (iii) dispersive?
(b) What distribution would be expected after two gen-
erations of growth in the
14
N-containing medium
assuming (i) conservative, (ii) semiconservative, or (iii)
dispersive replication?
ANS: (a) (i) One-half of the DNA molecules with
15
N in both
strands and the other half with
14
N in both strands; (ii) all
DNA molecules with one strand containing
15
N and the
complementary strand containing
14
N; (iii) all DNA
molecules with both strands containing roughly equal
amounts of
15
N and
14
N. (b) (i) 1/4 of the DNA molecules
with
15
N in both strands and 3/4 with
14
N in both strands;
(ii) half of the DNA molecules with one strand contain-
ing
15
N and the complementary strand containing
14
N
and the other half with
14
N in both strands; (iii) all DNA
molecules with both strands containing about 1/4
15
N
and 3/4
14
N.
10.3 Why do DNA molecules containing
15
N band at a differ-
ent position than DNA molecules containing
14
N when
centrifuged to equilibrium in 6M CsCl?
ANS:
15
Nitrogen contains eight neutrons instead of the seven
neutrons in the normal isotope of nitrogen,
14
N. There-
fore,
15
N has an atomic mass of about 15, whereas
14
N has
a mass of about 14. This difference means that purines
and pyrimidines containing
15
N have a greater density
(weight per unit volume) than those containing
14
N.
Equilibrium density-gradient centrifugation in 6M CsCl
separates DNAs or other macromolecules based on their
densities, and E. coli DNA, for example, that contains
15
N
has a density of 1.724 g/cm
2
, whereas E. coli DNA that
contains
14
N has a density of 1.710 g/cm
2
.
10.4 A DNA template plus primer with the structure
3´ P —TGCGAATTAGCGACAT— P 5´
5´ P —ATCGGTACGACGCTTAAC—OH 3´
...................
(where P a phosphate group) is placed in an in vitro
DNA synthesis system (Mg
2
, an excess of the four
deoxyribonucleoside triphosphates, etc.) containing a
mutant form of E. coli DNA polymerase I that lacks exo-
nuclease activity. The polymerase and exonuclease activ-
ities of this aberrant enzyme are identical to those of
normal E. coli DNA polymerase I. It simply has no exo-
nuclease activity.
(a) What will be the structure of the nal product?
(b) What will be the rst step in the reaction sequence?
ANS: (a)
3´ P -TGCGAATTAGCGACAT- P 5´
5´ P - ATCGGTACGACGCTTAATCGCTGTA-OH 3´;
........ ......... .............. ........
Note that DNA synthesis will not occur on the left end
since the 3′-terminus of the potential primer strand is
blocked with a phosphate group—all DNA polymerases
require a free 3′-OH terminus.
(b) The rst step will be the removal of the mismatched
C (exiting as dCMP) from the 3′-OH primer terminus
by the 3′ 5′ exonuclease (“proofreading”) activity.
10.5 How might continuous and discontinuous modes of
DNA replication be distinguished experimentally?
ANS: If nascent DNA is labeled by exposure to
3
H-thymidine
for very short periods of time, continuous replication
predicts that the label would be incorporated into chro-
mosome-sized DNA molecules, whereas discontinuous
replication predicts that the label would rst appear in
small pieces of nascent DNA (prior to covalent joining,
catalyzed by polynucleotide ligase).
10.6 E. coli cells contain ve different DNA polymerases—I,
II, III, IV, and V. Which of these enzymes catalyzes the
semiconservative replication of the bacterial chromo-
some during cell division? What are the functions of the
other four DNA polymerases in E. coli?
ANS: DNA polymerase III is the true replicase. DNA poly-
merase I removes the RNA primers and replaces them
with DNA. The other DNA polymerases play important
roles in DNA repair pathways (see Chapter 13).
10.7 The Boston barberry is an imaginary plant with a diploid
chromosome number of 4, and Boston barberry cells
are easily grown in suspended cell cultures.
3
H-thymi-
dine was added to the culture medium in which a
G1-stage cell of this plant was growing. After one cell
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 35 8/14/2015 6:42:53 PM
36-WC Answers to All Questions and Problems
generation of growth in
3
H-thymidine-containing
medium, colchicine was added to the culture medium.
The medium now contained both
3
H-thymidine and
colchicine. After two “generations” of growth in
3
H-thy-
midine-containing medium (the second “generation”
occurring in the presence of colchicine as well), the two
progeny cells (each now containing eight chromosomes)
were transferred to culture medium containing nonra-
dioactive thymidine (
3
H-thymidine) plus colchicine.
Note that a “generation” in the presence of colchicine
consists of a normal cell cycle’s chromosomal duplication
but no cell division. The two progeny cells were allowed
to continue to grow, proceeding through the “cell cycle,”
until each cell contained a set of metaphase chromo-
somes that looked like the following.
If autoradiography were carried out on these metaphase
chromosomes (four large plus four small), what pattern
of radioactivity (as indicated by silver grains on the auto-
radiograph) would be expected? (Assume no recombina-
tion between DNA molecules.)
ANS:
Two Plus two
For both the
lar
ge and small
chromosomes
10.8 Suppose that the experiment described in Problem 10.7
was carried out again, except this time replacing the
3
H-thymidine with nonradioactive thymidine at the
same time that the colchicine was added (after one cell
generation of growth in
3
H-thymidine-containing
medium). The cells were then maintained in colchicine
plus nonradioactive thymidine until the metaphase
shown in Problem 10.7 occurred. What would the auto-
radiographs of these chromosomes look like?
ANS:
Plus twoTwo For both the
lar
ge and small
chromosomes
10.9 Suppose that the DNA of cells (growing in a cell culture)
in a eukaryotic species was labeled for a short period of
time by the addition of
3
H-thymidine to the medium.
Next assume that the label was removed and the cells
were resuspended in nonradioactive medium. After a
short period of growth in nonradioactive medium, the
DNA was extracted from these cells, diluted, gently lay-
ered on lters, and autoradiographed. If autoradiographs
of the type
. . . . . . . . . . . .
were observed, what would this indicate about the nature
of DNA replication in these cells? Why?
ANS: The DNA replication was unidirectional rather than
bidirectional. As the intracellular pools of radioactive
3
H-thymidine are gradually diluted after transfer to non-
radioactive medium, less and less
3
H-thymidine will be
incorporated into DNA at each replicating fork. This
will produce autoradiograms with tails of decreasing
grain density at each growing point. Since such tails
appear at only one end of each track, replication must be
unidirectional. Bidirectional replication would produce
such tails at both ends of an autoradiographic track (see
Figure 10.31).
10.10 Arrange the following enzymes in the order of their
action during DNA replication in E. coli: (1) DNA poly-
merase I, (2) DNA polymerase III, (3) DNA primase,
(4) DNA gyrase, and (5) DNA helicase.
ANS: The correct sequence of action is 4, 5, 3, 2, 1.
10.11 Fifteen distinct DNA polymerases—a, b, g, d, e, k, z, h,
q, k, l, m, s, f, and Rev1—have been characterized in
mammals. What are the intracellular locations and func-
tions of these polymerases?
ANS: Current evidence suggests that polymerases a, d, and/or
e are required for the replication of nuclear DNA. Poly-
merase e is thought to catalyze the continuous synthesis
of the leading strand, and polymerases a and d are
thought to catalyze the replication of the lagging strand.
Polymerase a forms a complex with primase and initiates
the synthesis of Okazaki fragments during the discon-
tinuous replication of the lagging strand. Polymerase a
catalyzes the incorporation of approximately the rst 30
nucleotides in each Okazaki fragment before being
replaced by polymerase d, which then completes the syn-
thesis of the fragments. Polymerase g catalyzes replica-
tion of organellar chromosomes. Polymerases b, k, z, h,
q, k, l, m, s, f, and Rev1 function in various DNA repair
pathways (see Chapter 13).
10.12 The E. coli chromosome contains approximately 4 10
6
nucleotide pairs and replicates as a single bidirectional
replicon in approximately 40 minutes under a wide vari-
ety of growth conditions. The largest chromosome of
D. melanogaster contains about 6 10
7
nucleotide pairs.
(a) If this chromosome contains one giant molecule of
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 36 8/14/2015 6:42:54 PM
Answers to All Questions and Problems WC-37
DNA that replicates bidirectionally from a single origin
located precisely in the middle of the DNA molecule,
how long would it take to replicate the entire chromo-
some if replication in Drosophila occurred at the same
rate as replication in E. coli? (b) Actually, replication
rates are slower in eukaryotes than in prokaryotes. If
each replication bubble grows at a rate of 5000 nucleo-
tide pairs per minute in Drosophila and 100,000 nucleo-
tide pairs per minute in E. coli, how long will it take to
replicate the largest Drosophila chromosome if it contains
a single bidirectional replicon as described in (a) above?
(c) In Drosophila embryos, the nuclei divide every 9 to 10
minutes. Based on your calculations in (a) and (b) earlier,
what do these rapid nuclear divisions indicate about the
number of replicons per chromosome in Drosophila?
ANS: (a) Given bidirectional replication of a single replicon,
each replication fork must traverse 2 10
6
nucleotide
pairs in E. coli and 3 10
7
nucleotide pairs in the largest
Drosophila chromosome. If the rates were the same in
both species, it would take 15 times (3 10
7
/2 10
6
) as
long to replicate the Drosophila chromosome or 10 hours
(40 minutes 15 600 minutes).(b) If replication forks
in E. coli move 20 times as fast as replication forks in
Drosophila (100,000 nucleotide pairs per minute/5000
nucleotide pairs per minute), the largest Drosophila chromo-
some would require 8.3 days (10 hours 20 200 hours)
to complete one round of replication. (c) Each Drosophila
chromosome must contain many replicons in order to
complete replication in less than 10 minutes.
10.13 E. coli cells that have been growing in
14
N for many gen-
erations are transferred to medium containing only
15
N
and allowed to grow in this medium for four generations.
Their DNA is then extracted and analyzed by equilib-
rium CsCl density-gradient centrifugation. What pro-
portion of this DNA will band at the “light,” “hybrid,”
and “heavy” positions in the gradient?
ANS: No DNA will band at the “light” position; 12.5 percent
(2 of 16 DNA molecules) will band at the “hybrid” den-
sity; and 87.5 percent (14 of 16 DNA molecules) will
band at the “heavy” position.
10.14 The bacteriophage lambda chromosome has several
A:T-rich segments that denature when exposed to pH
11.05 for 10 minutes. After such partial denaturation,
the linear packaged form of the lambda DNA molecule
has the structure shown in Figure 10.9a. Following its
injection into an E. coli cell, the lambda DNA molecule
is converted into a covalently closed circular molecule by
hydrogen bonding between its complementary single-
stranded termini and the action of DNA ligase. It then
replicates as a q-shaped structure. The entire lambda
chromosome is 17.5 mm long. It has a unique origin of
replication located 14.3 mm from the left end of the lin-
ear form shown in Figure 10.9a. Draw the structure that
would be observed by electron microscopy after both
(1) replication of an approximately 6-mm-long segment
of the lambda chromosomal DNA molecule (in vivo) and
(2) exposure of this partially replicated DNA molecule to
pH 11.05 for 10 minutes (in vitro), (a) if replication had
proceeded bidirectionally from the origin, and (b) if rep-
lication had proceeded unidirectionally from the origin.
ANS:
(a) and (b)
b
b
b
c
c
c
d
d
d
e
f
g
h
e
f
g
h
a
a
j
i
or
h
g
f
e
i
j
a
i
j
10.15 What enzyme activity catalyzes each of the following
steps in the semiconservative replication of DNA in
prokaryotes?
(a) The formation of negative supercoils in progeny
DNA molecules. (b) The synthesis of RNA primers.
(c) The removal of RNA primers. (d) The covalent exten-
sion of DNA chains at the 3′-OH termini of primer
strands. (e) Proofreading of the nucleotides at the 3′-OH
termini of DNA primer strands?
ANS: (a) DNA gyrase; (b) primase; (c) the 5′ → 3′ exonuclease
activity of DNA polymerase I; (d) the 5′ → 3′ polymerase
activity of DNA polymerase III; (e) the 3′ → 5′ exonucle-
ase activity of DNA polymerase III.
10.16 One species of tree has a very large genome consisting of
2.0 10
10
base pairs of DNA.
(a) If this DNA was organized into a single linear mole-
cule, how long (meters) would this molecule be? (b) If
the DNA is evenly distributed among 10 chromosomes
and each chromosome has one origin of DNA replica-
tion, how long would it take to complete the S phase of
the cell cycle, assuming that DNA polymerase can syn-
thesize 2 10
4
bp of DNA per minute? (c) An actively
growing cell can complete the S phase of the cell cycle in
approximately 300 minutes. Assuming that the origins of
replication are evenly distributed, how many origins of
replication are present on each chromosome? (d) What
is the average number of base pairs between adjacent ori-
gins of replication?
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 37 8/14/2015 6:42:54 PM

38-WC Answers to All Questions and Problems
ANS: (a) (34 nm/100 bp)(2 10
10
bp) 6.8 10
9
6.8 meters.
(b) 2 10
10
/10 chromosomes 2 10
9
bp; 4 10
4
bp/
min (bidirectional); 2 10
9
/4 10
4
5 10
4
min
50,000 min. (c) (50,000 min)(1 ori) (300 min)
(X ori); X 50,000/300 167 ori. (d) (2 10
9
bp/
chrom.)/(167 ori/chrom.) 1.2 10
7
bp/ori.
10.17 Why must each of the giant DNA molecules in eukaryotic
chromosomes contain multiple origins of replication?
ANS: In eukaryotes, the rate of DNA synthesis at each replica-
tion fork is about 2500–3000 nucleotide pairs per min-
ute. Large eukaryotic chromosomes often contain
10
7
–10
8
nucleotide pairs. A single replication fork could
not replicate the giant DNA in one of these large chro-
mosomes fast enough to permit the observed cell gen-
eration times.
10.18 In E. coli, viable polA mutants have been isolated that
produce a defective gene product with little or no 5′→3′
polymerase activity, but normal 5′→3′ exonuclease activ-
ity. However, no polA mutant has been identied that
is completely decient in the 5′→3′ exonuclease activity,
while retaining 5′→3′ polymerase activity, of DNA poly-
merase I. How can these results be explained?
ANS: The 5′ → 3′ exonuclease activity of DNA polymerase I is
essential to the survival of the bacterium, whereas the
5′→3′ polymerase activity of the enzyme is not essential.
10.19 Other polA mutants of E. coli lack the 3′ → 5′ exonuclease
activity of DNA polymerase I. Will the rate of DNA syn-
thesis be altered in these mutants? What effect(s) will
these polA mutations have on the phenotype of the
organism?
ANS: No, the rate of DNA synthesis will not be altered. E. coli
strains carrying polA mutations that eliminate the 3′ → 5′
exonuclease activity of DNA polymerase I will exhibit
unusually high mutation rates.
10.20 Many of the origins of replication that have been charac-
terized contain AT-rich core sequences. Are these
AT-rich cores of any functional signicance? If so, what?
ANS: Because AT base pairs are held together by only two
hydrogen bonds instead of the three hydrogen bonds
present in GC base pairs, the two strands of AT-rich
regions of double helices are separated more easily, pro-
viding the single-stranded template regions required for
DNA replication.
10.21 (a) Why isn’t DNA primase activity required to initiate
rolling-circle replication?(b) DNA primase is required
for the discontinuous synthesis of the lagging strand,
which occurs on the single-stranded tail of the rolling
circle. Why?
ANS: Rolling-circle replication begins when an endonuclease
cleaves one strand of a circular DNA double helix. This
cleavage produces a free 3′-OH on one end of the cut
strand, allowing it to function as a primer. The discon-
tinuous synthesis of the lagging strand requires the de
novo initiation of each Okazaki fragment, which requires
DNA primase activity.
10.22 DNA polymerase I is needed to remove RNA primers
during chromosome replication in E. coli. However,
DNA polymerase III is the true replicase in E. coli. Why
does not DNA polymerase III remove the RNA
primers?
ANS: DNA polymerase III does not have a 5′ → 3′ exonuclease
activity that acts on double-stranded nucleic acids. Thus,
it cannot excise RNA primer strands from replicating
DNA molecules. DNA polymerase I is present in cells at
much higher concentrations and functions as a mono-
mer. Thus, DNA polymerase I is able to catalyze the
removal of RNA primers from the vast number of Oka-
zaki fragments formed during the discontinuous replica-
tion of the lagging strand.
10.23 In E. coli, three different proteins are required to unwind
the parental double helix and keep the unwound strands
in an extended template form. What are these proteins,
and what are their respective functions?
ANS: DNA helicase unwinds the DNA double helix, and sin-
gle-strand DNA-binding protein coats the unwound
strands, keeping them in an extended state. DNA gyrase
catalyzes the formation of negative supercoiling in E. coli
DNA, and this negative supercoiling behind the replica-
tion forks is thought to drive the unwinding process
because superhelical tension is reduced by unwinding
the complementary strands.
10.24 How similar are the structures of DNA polymerase I and
DNA polymerase III in E. coli? What is the structure of
the DNA polymerase III holoenzyme? What is the func-
tion of the dnaN gene product in E. coli?
ANS: DNA polymerase I is a single polypeptide of molecular
weight 109,000, whereas DNA polymerase III is a
complex multimeric protein. The DNA polymerase III
holoenzyme has a molecular mass of about 900,000
daltons and is composed of at least 20 different poly-
peptides. The dnaN gene product, the b subunit of
DNA polymerase III, forms a dimeric clamp that encir-
cles the DNA molecule and prevents the enzyme from
dissociating from the template DNA during replication.
10.25 The dnaA gene product of E. coli is required for the ini-
tiation of DNA synthesis at oriC. What is its function?
How do we know that the DnaA protein is essential to
the initiation process?
ANS: DnaA protein initiates the formation of the replication
bubble by binding to the 9-bp repeats of OriC. DnaA
protein is known to be required for the initiation process
because bacteria with temperature-sensitive mutations in
the dnaA gene cannot initiate DNA replication at restric-
tive temperatures.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 38 8/14/2015 6:42:54 PM

Answers to All Questions and Problems WC-39
10.26 What is a primosome, and what are its functions? What
essential enzymes are present in the primosome? What
are the major components of the E. coli replisome? How
can geneticists determine whether these components are
required for DNA replication?
ANS: The primosome is a protein complex that initiates the
synthesis of Okazaki fragments during lagging strand
synthesis. The major components of the E. coli DNA
primosome are DNA primase and DNA helicase. Genet-
icists have been able to show that both DNA primase
and DNA helicase are required for DNA replication by
demonstrating that mutations in the genes encoding
these enzymes result in the arrest of DNA synthesis in
mutant cells under conditions where the altered proteins
are inactive.
10.27 The chromosomal DNA of eukaryotes is packaged into
nucleosomes during the S phase of the cell cycle. What
obstacles do the size and complexity of both the repli-
some and the nucleosome present during the semicon-
servative replication of eukaryotic DNA? How might
these obstacles be overcome?
ANS: Nucleosomes and replisomes are both large macromo-
lecular structures, and the packaging of eukaryotic
DNA into nucleosomes raises the question of how a
replisome can move past a nucleosome and replicate
the DNA in the nucleosome in the process. The most
obvious solution to this problem would be to com-
pletely or partially disassemble the nucleosome to
allow the replisome to pass. The nucleosome would
then reassemble after the replisome had passed. One
popular model has the nucleosome partially disassem-
bling, allowing the replisome to move past it (see
Figure 10.33b).
10.28 Two mutant strains of E. coli each have a temperature-
sensitive mutation in a gene that encodes a product
required for chromosome duplication. Both strains repli-
cate their DNA and divide normally at 25°C but are
unable to replicate their DNA or divide at 42°C. When
cells of one strain are shifted from growth at 25°C to
growth at 42°C, DNA synthesis stops immediately. When
cells of the other strain are subjected to the same tempera-
ture shift, DNA synthesis continues, albeit at a decreasing
rate, for about a half hour. What can you conclude about
the functions of the products of these two genes?
ANS: The product of the rst gene is required for DNA chain
extension, whereas the product of the second gene is
only required for the initiation of DNA synthesis.
10.29 In what ways does chromosomal DNA replication in
eukaryotes differ from DNA replication in prokaryotes?
ANS: (1) DNA replication usually occurs continuously in
rapidly growing prokaryotic cells but is restricted to
the S phase of the cell cycle in eukaryotes. (2) Most
eukaryotic chromosomes contain multiple origins of
replication, whereas most prokaryotic chromosomes
contain a single origin of replication. (3) Prokaryotes
utilize two catalytic complexes that contain the same
DNA polymerase to replicate the leading and lagging
strands, whereas eukaryotes utilize two or three distinct
DNA polymerases for leading and lagging strand syn-
thesis. (4) Replication of eukaryotic chromosomes
requires the partial disassembly and reassembly of
nucleosomes as replisomes move along parental DNA
molecules. In prokaryotes, replication probably involves
a similar partial disassembly/reassembly of nucleo-
some-like structures. (5) Most prokaryotic chromo-
somes are circular and thus have no ends. Most
eukaryotic chromosomes are linear and have unique
termini called telomeres that are added to replicating
DNA molecules by a unique, RNA-containing enzyme
called telomerase.
10.30 (a) The chromosome of the bacterium Salmonella
typhimurium contains about 4 10
6
nucleotide pairs.
Approximately how many Okazaki fragments are pro-
duced during one complete replication of the S.
typhimurium chromosome? (b) The largest chromosome
of D. melanogaster contains approximately 6 10
7
nucle-
otide pairs. About how many Okazaki fragments are pro-
duced during the replication of this chromosome?
ANS: (a) 2000–4000 Okazaki fragments. (b) 300,000–600,000
Okazaki fragments.
10.31 In the yeast S. cerevisiae, haploid cells carrying a mutation
called est1 (for ever-shorter telomeres) lose distal telo-
mere sequences during each cell division. Predict the
ultimate phenotypic effect of this mutation on the prog-
eny of these cells.
ANS: The chromosomes of haploid yeast cells that carry the
est1 mutation become shorter during each cell division.
Eventually, chromosome instability results from the
complete loss of telomeres, and cell death occurs because
of the deletion of essential genes near the ends of
chromosomes.
10.32 Assume that the sequence of a double-stranded DNA
shown below is present at one end of a large DNA mol-
ecule in a eukaryotic chromosome.
5′-(centromere sequence)-gattccccgggaagcttggggggcccatcttcgtacgtctttgca-3′
3′-(centromere sequence)-ctaaggggcccttcgaaccccccgggtagaagcatgagaaacgt-5′
You have reconstituted a eukaryotic replisome that is
active in vitro. However, it lacks telomerase activity. If
you isolate the DNA molecule shown above and repli-
cate it in your in vitro system, what products would you
expect?
ANS: Without telomerase, the 5′ end of the newly replicated
strand will be missing some bases. The exact number of
missing bases does not matter:
5′-(centromere sequence)-gattccccgggaagcttggggggcccatcttcgtacgtctttgca-3′
3′-(centromere sequence)-ctaaggggcccttcgaaccccccgcgggtagaagcatg-5′
5′-(centromere sequence)-gattccccgggaagcttggggggcccatcttcgtacgtctttgca-3′
3′-(centromere sequence)-ctaaggggcccttc-5′
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 39 8/14/2015 6:42:54 PM

40-WC Answers to All Questions and Problems
Chapter 11
11.1 Distinguish between DNA and RNA (a) chemically,
(b) functionally, and (c) by location in the cell.
ANS: (a) RNA contains the sugar ribose, which has a hydroxyl
(OH) group on the 2-carbon; DNA contains the sugar
2-deoxyribose, with only hydrogens on the 2-carbon.
RNA usually contains the base uracil at positions where
thymine is present in DNA. However, some DNAs con-
tain uracil, and some RNAs contain thymine. DNA exists
most frequently as a double helix (double-stranded mole-
cule); RNA exists more frequently as a single-stranded
molecule. But, some DNAs are single-stranded and some
RNAs are double-stranded. (b) The main function of
DNA is to store genetic information and to transmit that
information from cell to cell and from generation to gen-
eration. RNA stores and transmits genetic information in
some viruses that contain no DNA. In cells with both
DNA and RNA: (1) mRNA acts as an intermediary in pro-
tein synthesis, carrying the information from DNA in the
chromosomes to the ribosomes (sites at which proteins are
synthesized). (2) tRNAs carry amino acids to the ribo-
somes and function in codon recognition during the syn-
thesis of polypeptides. (3) rRNA molecules are essential
components of the ribosomes. (4) snRNAs are important
components of spliceosomes. (5) miRNAs play key roles
in regulating gene expression (see Chapter 18). (c) DNA is
located primarily in the chromosomes, which are found in
the nucleus of eukaryotic cells; however, some DNA is
also found in cytoplasmic organelles, such as mitochon-
dria and chloroplasts. RNA is located throughout cells.
11.2 What bases in the mRNA transcript would represent the
following DNA template sequence: 5′-TGCAGACA-3′?
ANS: 3′-ACGUCUGU-5′
11.3 What bases in the transcribed strand of DNA would give
rise to the following mRNA base sequence:
5′-CUGAU-3′?
ANS: 3′-GACTA-5′
11.4 On the basis of what evidence was the messenger RNA
hypothesis established?
ANS: The genetic information of cells is stored in DNA, which
is located predominantly in the chromosomes. The gene
products (polypeptides) are synthesized primarily in the
cytoplasm on ribosomes. Some intermediate must there-
fore carry the genetic information from the chromo-
somes to the ribosomes. RNA molecules (mRNAs) were
shown to perform this function by means of RNA pulse-
labeling and pulse-chase experiments combined with
autoradiography. The enzyme RNA polymerase was
subsequently shown to catalyze the synthesis of mRNA
using chromosomal DNA as a template. Finally, the
mRNA molecules synthesized by RNA polymerase were
shown to faithfully direct the synthesis of specic poly-
peptides when used in in vitro protein synthesis systems.
11.5 At what locations in a eukaryotic cell does protein syn-
thesis occur?
ANS: Protein synthesis occurs on ribosomes. In eukaryotes,
most of the ribosomes are located in the cytoplasm and
are attached to the extensive membranous network of
endoplasmic reticulum. Some protein synthesis also
occurs in cytoplasmic organelles such as chloroplasts and
mitochondria.
11.6 List three ways in which the mRNAs of eukaryotes differ
from the mRNAs of prokaryotes.
ANS: (1) Eukaryotes have a 5′ cap on their mRNAs; prokary-
otes do not. (2) Messenger RNAs of eukaryotes generally
have a 3′ poly-A tail, prokaryotic mRNAs do not. (3)
Messenger RNA formation in eukaryotes involves
removal of introns (when present) and the splicing
together of exons. Prokaryotic genes (with very rare
exceptions) do not have introns.
11.7 What different types of RNA molecules are present in
prokaryotic cells? in eukaryotic cells? What roles do
these different classes of RNA molecules play in the cell?
ANS: Both prokaryotic and eukaryotic organisms contain mes-
senger RNAs, transfer RNAs, and ribosomal RNAs. In
addition, eukaryotes contain small nuclear RNAs and
micro RNAs. Messenger RNA molecules carry genetic
information from the chromosomes (where the informa-
tion is stored) to the ribosomes in the cytoplasm (where
the information is expressed during protein synthesis).
The linear sequence of triplet codons in an mRNA mol-
ecule species the linear sequence of amino acids in the
polypeptides produced during translation of that mRNA.
Transfer RNA molecules are small (about 80 nucleotides
long) molecules that carry amino acids to the ribosomes
and provide the codon-recognition specicity during
translation. Ribosomal RNA molecules provide part of
the structure and function of ribosomes; they represent
an important part of the machinery required for the syn-
thesis of polypeptides. Small nuclear RNAs are struc-
tural components of spliceosomes, which excise
noncoding intron sequences from nuclear gene tran-
scripts. Micro RNAs are involved in the regulation of
gene expression.
11.8 Many eukaryotic genes contain noncoding introns that
separate the coding sequences or exons of these genes. At
what stage during the expression of these split genes are
the noncoding intron sequences removed?
ANS: The entire nucleotide-pair sequences—including the
introns—of the genes are transcribed by RNA poly-
merase to produce primary transcripts that still contain
the intron sequences. The intron sequences are then
spliced out of the primary transcripts to produce the
mature, functional RNA molecules. In the case of
protein-encoding nuclear genes of higher eukaryotes,
the introns are spliced out by complex macromolecular
structures called spliceosomes.
11.9 For several decades, the dogma in biology has been that
molecular reactions in living cells are catalyzed by
enzymes composed of polypeptides. We now know that
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 40 8/14/2015 6:42:54 PM

Answers to All Questions and Problems WC-41
the introns of some precursor RNA molecules such as the
rRNA precursors in Tetrahymena are removed autocata-
lytically (self-spliced) with no involvement of any cata-
lytic protein. What does the demonstration of autocatalytic
splicing indicate about the dogma that biological reac-
tions are always catalyzed by proteinaceous enzymes?
ANS: “Self-splicing” of RNA precursors demonstrates that
RNA molecules can also contain catalytic sites; this
property is not restricted to proteins.
11.10 What role(s) do spliceosomes play in pathways of gene
expression? What is their macromolecular structure?
ANS: Spliceosomes excise intron sequences from nuclear gene
transcripts to produce the mature mRNA molecules that
are translated on ribosomes in the cytoplasm. Spliceo-
somes are complex macromolecular structures composed
of snRNA and protein molecules (see Figure 11.22).
11.11 What components of the introns of nuclear genes that
encode proteins in higher eukaryotes are conserved and
required for the correct excision of intron sequences
from primary transcripts by spliceosomes?
ANS: The introns of protein-encoding nuclear genes of higher
eukaryotes almost invariably begin (5′) with GT and end
(3′) with AG. In addition, the 3′ subterminal A in the
“TACTAAC box” is completely conserved; this A is
involved in bond formation during intron excision.
11.12 Match one of the following terms with each of the
descriptions given below. Terms: (1) sigma (s) factor;
(2) poly(A) tail; (3) TATAAT; (4) exons; (5) TATAAAA;
(6) RNA polymerase III; (7) intron; (8) RNA poly-
merase II; (9) heterogeneous nuclear RNA (hnRNA);
(10) snRNA; (11) RNA polymerase I; (12) TTGACA;
(13) GGCCAATCT (CAAT box).
Descriptions:
(a) Intervening sequence found in many eukaryotic
genes.
(b) A conserved nucleotide sequence (-30) in eukaryotic
promoters involved in the initiation of transcription.
(c) Small RNA molecules that are located in the nuclei of
eukaryotic cells, most as components of the spliceosome,
that participate in the excision of introns from nuclear
gene transcripts.
(d) A sequence (-10) in the nontemplate strand of the
promoter of E. coli that facilitates the localized unwind-
ing of DNA when complexed with RNA polymerase.
(e) The RNA polymerase in the nucleus that catalyzes
the synthesis of all rRNAs except for the small 5S rRNA.
(f) The subunit of prokaryotic RNA polymerase that is
responsible for the initiation of transcription at
promoters.
(g) An E. coli promoter sequence located 35 nucleotides
upstream from the transcription-initiation site; it serves
as a recognition site for the sigma factor.
(h) The RNA polymerase in the nucleus that catalyzes
the synthesis of the transfer RNA molecules and small
nuclear RNAs.
(i) A polyadenosine tract 20–200 nucleotides long that is
added to the 3′ end of most eukaryotic messenger RNAs.
(j) The RNA polymerase that transcribes nuclear genes
that encode proteins.
(k) A conserved sequence in the nontemplate strand of
eukaryotic promoters that is located about 80 nucleo-
tides upstream from the transcription start site.
(l) Segments of an eukaryotic gene that correspond to
the sequences in the nal processed RNA transcript of
the gene.
(m) The population of primary transcripts in the nucleus
of a eukaryotic cell.
ANS: (a) 7; (b) 5; (c) 10; (d) 3; (e) 11; (f) 1; (g) 12; (h) 6; (i) 2;
(j) 8; (k) 13; (l) 4; (m) 9.
11.13 (a) Which of the following nuclear pre-mRNA nucleo-
tide sequences potentially contains an intron?
(1) 5′-UGACCAUGGCGCUAACACUGCCAAU
UGGCAAUACUGACCUGAUAGCAUCAGCCAA-3′
(2) 5′-UAGUCUCAUCUGUCCAUUGACUUCGAAA
CUGAAUCGUAACUCCUACGUCUAUGGA-3′
(3) 5′-UAGCUGUUUGUCAUGACUGACUGGUCA
CUAUCGUACUAACCUGUCAUGCAAUGUC-3′
(4) 5′-UAGCAGUUCUGUCGCCUCGUGGUGCUG
CUGGCCCUUCGUCGCUCGGGCUUAGCUA-3′
(5) 5′-UAGGUUCGCAUUGACGUACUUCUGAAAC
UACUAACUACUAACGCAUCGAGUCUCAA-3′
(b) One of the ve pre-mRNAs shown in (a) may undergo
RNA splicing to excise an intron sequence. What mRNA
nucleotide sequence would be expected to result from
this splicing event?
ANS: (a) Sequence 5. It contains the conserved intron sequences:
a 5′ GU, a 3′ AG, and a UACUAAC internal sequence
providing a potential bonding site for intron excision.
Sequence 4 has a 5′ GU and a 3′ AG but contains no
internal A for the bonding site during intron excision.
(b) 5′—UAGUCUCAA—3′; the putative intron from the
5′ GU through the 3′ AG has been removed.
11.14 What is the function of the introns in eukaryotic genes?
ANS: This is a wide-open question at present! There is much
speculation, but little hard evidence. One popular
hypothesis is that introns enhance exon shufing by
increasing recombination events between sequences
encoding adjacent domains of a polypeptide. Also, in one
yeast mitochondrial gene, the introns contain open read-
ing frames that encode “maturases” that splice out these
introns—a neat negative feedback control. Other introns
may be merely relics of evolution.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 41 8/14/2015 6:42:54 PM
42-WC Answers to All Questions and Problems
11.15 A particular gene is inserted into the phage lambda chro-
mosome and is shown to contain three introns.
(a) The primary transcript of this gene is puried from
isolated nuclei. When this primary transcript is hybrid-
ized under R-loop conditions with the recombinant
lambda chromosome carrying the gene, what will the
R-loop structure(s) look like? Label your diagram.
(b) The mRNA produced from the primary transcript of
this gene is then isolated from cytoplasmic polyribosomes
and similarly examined by the R-loop hybridization
procedure using the recombinant lambda chromosome
carrying the gene. Diagram what the R-loop structure(s)
will look like when the cytoplasmic mRNA is used. Again,
label the components of your diagram.
ANS:
(a)
(b)
Displaced single-stranded DNA ("R-loop")
Primary transcript
mRNA
Exon1
Exon1
Intron1
Intron2
Exon2
Exon2
Intron3
Exon3
Exon3
Exon4
Exon4
λ DNA
Intron1
Intron2 Intron3
λ
DNA
Displaced single-stranded exon DNA ("R-loops")
λ
DNA
λDNA
11.16 A segment of DNA in E. coli has the following sequence
of nucleotide pairs:
3´-A
5´-T TTT
TTTTTTTTT
AAAAAAAAA
AAA
G
GGGGG
GGGG
CCCC
CCCCCCG-5´
C-
3´
When this segment of DNA is transcribed by RNA poly-
merase, what will be the sequence of nucleotides in the
RNA transcript if the promoter is located to the left of
the sequence shown?
ANS: If there is a promoter located upstream from this DNA
segment, the nucleotide sequence of this portion of the
RNA transcript will be
5′-UACGAUGACGAUAAGCGACAUAGC-3′. If there
is no upstream promoter, this segment of DNA will not
be transcribed.
11.17 A segment of DNA in E. coli has the following sequence
of nucleotide pairs:
3´-A
5´
-T TTT
TTTTGATTT
AAAACTAAA
AAA
G
GCGGA
GGGA
CCCT
CCGCCTG-
C-
TTT
TTTTTTTTTA
AAAAAAAAAT
AAA
G
GGGGG
GGGG
CCCC
CCCCCC G-5´
C-3´
When this segment of DNA is transcribed by RNA poly-
merase, what will be the sequence of nucleotides in the
RNA transcript?
ANS: Assuming that there is a 35 sequence upstream from
the consensus 10 sequence in this segment of the DNA
molecule, the nucleotide sequence of the transcript will
be 5′-ACCCGACAUAGCUACGAUGACGAUAAGC
GACAUAGC-3′.
11.18 A segment of DNA in E. coli has the following sequence
of nucleotide pairs:
3´-A
5´
-T AGA
GCTTAGCGA
CGAATCGCT
TCT
TAGATA
GCGC
CGCG
ATCTATT
A
A
T
T
A
T
A
A
T
C
G
T-
A-
AAC
ACTATGGTCG
TGATACCAGC
TTG
G
TGACT
GGCA
CCGT
C
A
T
C
G
G
C
A
T
T
AACTG
AT
-5´
A-3´
When this segment of DNA is transcribed by RNA poly-
merase, what will be the sequence of nucleotides in the
RNA transcript?
ANS: Given the consensus 35 and 10 sequences in this seg-
ment of DNA and the fact that transcripts almost always
start with a purine, the predicted nucleotide sequence of
the transcript is 5′-ACCCGACAUAGCUACGAUGA
CGAUA-3′.
11.19 A segment of human DNA has the following sequence of
nucleotide pairs:
3´-A
5´
-T AGA
GCTTAGCTT
CGAATCGAA
TCT
TAGATA
GCGA
CGCT
ATCTATT
A
A
T
G
C
G
C
A
T
C
G
T-
A-
AAC
ACTATGGTCG
TGATACCAGC
TTG
G
TGACT
GGCA
CCGT
C
A
T
C
G
G
C
A
T
T
AACTG
AT
-5´
A-3´
When this segment of DNA is transcribed by RNA poly-
merase, what will be the sequence of nucleotides in the
RNA transcript?
ANS: Assuming that there is a CAAT box located upstream
from the TATA box shown in this segment of DNA,
the nucleotide sequence of the transcript will be
5′-ACCCGACAUAGCUACGAUGACGAUA-3′.
11.20 The genome of a human must store a tremendous amount
of information using the four nucleotide pairs present in
DNA. What does the language of computers tell us about
the feasibility of storing large amounts of information
using an alphabet composed of just four letters?
ANS: Given the vast amount of information that can be stored
on a small computer chip by using a binary code, it is
clear that large quantities of genetic information can be
stored in the genomes of organisms by using the four-
letter alphabet of the genetic code.
11.21 What is the central dogma of molecular genetics? What
impact did the discovery of RNA tumor viruses have on
the central dogma?
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 42 8/14/2015 6:42:55 PM

Answers to All Questions and Problems WC-43
ANS: According to the central dogma, genetic information is
stored in DNA and is transferred from DNA to RNA to
protein during gene expression. RNA tumor viruses
store their genetic information in RNA, and that infor-
mation is copied into DNA by the enzyme reverse tran-
scriptase after a virus infects a host cell. Thus, the
discovery of RNA tumor viruses or retroviruses—retro
for backwards ow of genetic information—provided an
exception to the central dogma.
11.22 The biosynthesis of metabolite X occurs via six steps
catalyzed by six different enzymes. What is the minimal
number of genes required for the genetic control of this
metabolic pathway? Might more genes be involved?
Why?
ANS: If six different enzymes are required for the pathway,
then minimally six genes are necessary for genetic con-
trol of the pathway. However, because the expression of
these enzymes may rely on the product of other genes,
such as transcription factors, more than six genes could
be involved in genetic control of this pathway.
11.23 What do processes of DNA synthesis, RNA synthesis,
and polypeptide synthesis have in common?
ANS: DNA, RNA, and protein synthesis all involve the synthe-
sis of long chains of repeating subunits. All three pro-
cesses can be divided into three stages: chain initiation,
chain elongation, and chain termination.
11.24 What are the two stages of gene expression? Where do
they occur in a eukaryotic cell? a prokaryotic cell?
ANS: The two stages of gene expression are as follows:
(1) Transcription—the transfer of genetic information
from DNA to RNA.
(2) Translation—the transfer of genetic information from
RNA to protein. In eukaryotes, transcription occurs in
the nucleus and translation occurs in the cytoplasm
on complex macromolecular structures called ribosomes.
In prokaryotes, transcription and translation are often
coupled with mRNA molecules often being translated
by ribosomes while still being synthesized during
transcription.
11.25 Compare the structures of primary transcripts with those
of mRNAs in prokaryotes and eukaryotes. On average, in
which group of organisms do they differ the most?
ANS: The primary transcripts of eukaryotes undergo more
extensive posttranscriptional processing than those of
prokaryotes. Thus, the largest differences between
mRNAs and primary transcripts occur in eukaryotes.
Transcript processing is usually restricted to the excision
of terminal sequences in prokaryotes. In contrast, eukary-
otic transcripts are usually modied by (1) the excision of
intron sequences; (2) the addition of 7-methyl guanosine
caps to the 5′ termini; (3) the addition of poly(A) tails to
the 3′ termini. In addition, the sequences of some eukary-
otic transcripts are modied by RNA editing processes.
11.26 What ve types of RNA molecules participate in the
process of gene expression? What are the functions of
each type of RNA? Which types of RNA perform their
function(s) in (a) the nucleus and (b) the cytoplasm?
ANS: The ve types of RNA molecules that are involved in
gene expression are mRNAs, rRNAs, tRNAs, micro
RNAs, and snRNAs. mRNA molecules carry genetic
information from genes to the sites of protein synthesis
and specify the amino acid sequences of polypeptides.
rRNAs are major structural components of the ribo-
somes and provide functions required for translation.
tRNA molecules are the adapters that provide amino
acid-codon specicity during translation; each tRNA is
activated by a specic amino acid and contains an antico-
don sequence that is complementary or partially comple-
mentary to one, two, or three codons in mRNAs.
Micro-RNAs are involved in regulative gene expression
(see Chapter 18). snRNAs are structural components of
the spliceosomes that excise introns from gene transcripts
in eukaryotes. snRNAs perform their splicing functions
in the nucleus. mRNAs carry information from the
nucleus to the cytoplasm, so they function in both com-
partments of the cell. However, their most prominent
function is to direct the synthesis of polypeptides during
translation, which occurs in the cytoplasm. rRNAs and
tRNAs perform their functions during translation in the
cytoplasm. Micro-RNAs become incorporated into ribo-
nucleoprotein complexes in the cytoplasm.
11.27 Why was the need for an RNA intermediary in protein
synthesis most obvious in eukaryotes? How did research-
ers rst demonstrate that RNA synthesis occurred in the
nucleus and that protein synthesis occurred in the
cytoplasm?
ANS: In eukaryotes, the genetic information is stored in DNA
in the nucleus, whereas proteins are synthesized on ribo-
somes in the cytoplasm. How could the genes, which are
separated from the sites of protein synthesis by a double-
membrane—the nuclear envelope, direct the synthesis of
polypeptides without some kind of intermediary to carry
the specications for the polypeptides from the nucleus
to the cytoplasm? Researchers rst used labeled RNA
and protein precursors and autoradiography to demon-
strate that RNA synthesis and protein synthesis occurred
in the nucleus and the cytoplasm, respectively.
11.28 Two eukaryotic genes encode two different polypeptides,
each of which is 335 amino acids long. One gene con-
tains a single exon; the other gene contains an intron of
41,324 nucleotide pairs long. Which gene would you
expect to be transcribed in the least amount of time?
Why? When the mRNAs specied by these genes are
translated, which mRNA would you expect to be trans-
lated in the least time? Why?
ANS: Because transcription results in a primary transcript from
the DNA template, the DNA sequence for the single-
exon gene is the shortest, and so it will be transcribed in
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 43 8/14/2015 6:42:55 PM

44-WC Answers to All Questions and Problems
the least amount of time. However, because each poly-
peptide is the same length, the mature mRNAs for both
genes will be the same length and will be translated in
the same amount of time.
11.29 Design an experiment to demonstrate that RNA tran-
scripts are synthesized in the nucleus of eukaryotes and
are subsequently transported to the cytoplasm.
ANS: A simple pulse- and pulse/chase-labeling experiment will
demonstrate that RNA is synthesized in the nucleus and
is subsequently transported to the cytoplasm. This
experiment has two parts: (1) pulse label eukaryotic cul-
ture cells by growing them in
3
H-uridine for a few min-
utes and localize the incorporated radioactivity by
autoradiography. (2) Repeat the experiment, but this
time add a large excess of nonradioactive uridine to the
medium in which the cells are growing after the labeling
period and allow the cells to grow in the nonradioactive
medium for about an hour. Then localize the incorpo-
rated radioactivity by autoradiography.
11.30 Total RNA was isolated from human cells growing in cul-
ture. This RNA was mixed with nontemplate strands
(single strands) of the human gene encoding the enzyme
thymidine kinase, and the RNA–DNA mixture was incu-
bated for 12 hours under renaturation conditions. Would
you expect any RNA–DNA duplexes to be formed during
the incubation? If so, why? If not, why not? The same
experiment was then performed using the template strand
of the thymidine kinase gene. Would you expect any
RNA–DNA duplexes to be formed in this second
experiment? If so, why? If not, why not?
ANS: RNA–DNA duplexes will be formed when the template
strand is used, but not when the nontemplate strand is
used. (However, if some hybridization is observed with
the nontemplate strand, this is because total RNA was
used and is nonspecic to the thymidine kinase gene)
Only one strand—the template strand—of most genes is
transcribed. Thus, RNA will contain nucleotide sequences
complementary to the template strand but not to the
nontemplate strand.
11.31 Two preparations of RNA polymerase from E. coli are
used in separate experiments to catalyze RNA synthesis
in vitro using a puried fragment of DNA carrying the
argH gene as template DNA. One preparation catalyzes
the synthesis of RNA chains that are highly heteroge-
neous in size. The other preparation catalyzes the syn-
thesis of RNA chains that are all the same length. What
is the most likely difference in the composition of the
RNA polymerases in the two preparations?
ANS: The rst preparation of RNA polymerase is probably
lacking the sigma subunit and, as a result, initiates the
synthesis of RNA chains at random sites along both
strands of the argH DNA. The second preparation prob-
ably contains the sigma subunit and initiates RNA chains
only at the site used in vivo, which is governed by the
position of the 10 and 35 sequences of the promoter.
11.32 Transcription and translation are coupled in prokaryotes.
Why is this not the case in eukaryotes?
ANS: In eukaryotes, transcription occurs in the nucleus and
translation occurs in the cytoplasm. Because these pro-
cesses occur in different compartments of the cell, they
cannot be coupled as they are in prokaryotes.
11.33 What two elements are almost always present in the
promoters of eukaryotic genes that are transcribed by
RNA polymerase II? Where are these elements located
relative to the transcription start site? What are their
functions?
ANS: TATA and CAAT boxes. The TATA and CAAT boxes are
usually centered at positions 30 and 80, respectively,
relative to the startpoint (1) of transcription. The
TATA box is responsible for positioning the transcrip-
tion startpoint; it is the binding site for the rst basal
transcription factor that interacts with the promoter.
The CAAT box enhances the efciency of transcrip-
tional initiation.
11.34 In what ways are most eukaryotic gene transcripts modi-
ed? What are the functions of these posttranscriptional
modications?
ANS: (1) Intron sequences are spliced out of gene transcripts to
provide contiguous coding sequences for translation. (2)
The 7-methyl guanosine caps added to the 5′ termini of
most eukaryotic mRNAs help protect them from degra-
dation by nucleases and are recognized by proteins
involved in the initiation of translation. (3) The poly(A)
tails at the 3′ termini of mRNAs play an important role
in their transport from the nucleus to the cytoplasm and
enhance their stability.
11.35 How does RNA editing contribute to protein diversity in
eukaryotes?
ANS: RNA editing sometimes leads to the synthesis of two or
more distinct polypeptides from a single mRNA.
11.36 How do the mechanisms by which the introns of tRNA
precursors, Tetrahymena rRNA precursors, and nuclear
pre-mRNAs are excised differ? In which process are
snRNAs involved? What role(s) do these snRNAs
play?
ANS: The introns of tRNA precursors, Tetrahymena rRNA
precursors, and nuclear pre-mRNAs are excised by com-
pletely different mechanisms. (1) Introns in tRNA are
excised by cleavage and joining events catalyzed by splic-
ing nucleases and ligases, respectively. (2) Introns in Tet-
rahymena rRNA precursors are excised autocatalytically.
(3) Introns of nuclear pre-mRNAs are excised by spli-
ceosomes. snRNAs are involved in nuclear pre-mRNA
splicing as structural components of spliceosomes. In
addition, snRNA U1 is required for the cleavage events
at the 5′ termini of introns; U1 is thought to base-pair
with a partially complementary consensus sequence at
this position in pre-mRNAs.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 44 8/14/2015 6:42:55 PM

Answers to All Questions and Problems WC-45
11.37 A mutation in an essential human gene changes the
5′ splice site of a large intron from GT to CC. Predict
the phenotype of an individual homozygous for this
mutation.
ANS: This zygote will probably be nonviable because the gene
product is essential, and the elimination of the 5′ splice
site will almost certainly result in the production of a
nonfunctional gene product.
11.38 Total RNA was isolated from nuclei of human cells
growing in culture. This RNA was mixed with a puried,
denatured DNA fragment that carried a large intron of a
housekeeping gene (a gene expressed in essentially all
cells), and the RNA–DNA mixture was incubated for
12 hours under renaturation conditions. Would you
expect any RNA–DNA duplexes to be formed during the
incubation? If so, why? If not, why not? The same exper-
iment was then performed using total cytoplasmic RNA
from these cells. Would you expect any RNA–DNA
duplexes to be formed in this second experiment? If so,
why? If not, why not?
ANS: In the rst experiment, yes, some hybridization would be
expected because not all of the RNA in the preparation
has been completely processed and will still contain the
intron sequence. However, if only cytoplasmic RNA is
used in the hybridization experiment, all of the mRNA in
the preparation will have been processed (because pro-
cessing occurs in the nucleus) and no hybridization will
be observed.
Chapter 12
12.1 In a general way, describe the molecular organization of
proteins and distinguish proteins from DNA, chemically
and functionally. Why is the synthesis of proteins of par-
ticular interest to geneticists?
ANS: Proteins are long chainlike molecules made up of amino
acids linked together by peptide bonds. Proteins are com-
posed of carbon, hydrogen, nitrogen, oxygen, and usually
sulfur. They provide the enzymatic capacity and much of
the structure of living organisms. DNA is composed
of phosphate, the pentose sugar 2-deoxyribose, and four
nitrogen-containing organic bases (adenine, cytosine,
guanine, and thymine). DNA stores and transmits the
genetic information in most living organisms. Protein
synthesis is of particular interest to geneticists because
proteins are the primary gene products—the key inter-
mediates through which genes control the phenotypes of
living organisms.
12.2 At what locations in the cell does protein synthesis occur?
ANS: Protein synthesis occurs on ribosomes. In eukaryotes,
most of the ribosomes are located in the cytoplasm and
are attached to the extensive membranous network of
endoplasmic reticulum. Some protein synthesis also
occurs in cytoplasmic organelles such as chloroplasts and
mitochondria.
12.3 Is the number of potential alleles of a gene directly
related to the number of nucleotide pairs in the gene? Is
such a relationship more likely to occur in prokaryotes or
in eukaryotes? Why?
ANS: It depends on how you dene alleles. If every variation in
nucleotide sequence is considered to be a different allele,
even if the gene product and the phenotype of the organ-
ism carrying the mutation are unchanged, then the
number of alleles will be directly related to gene size.
However, if the nucleotide sequence change must pro-
duce an altered gene product or phenotype before it is
considered a distinct allele, then there will be a positive
correlation, but not a direct relationship, between the
number of alleles of a gene and its size in nucleotide
pairs. The relationship is more likely to occur in pro-
karyotes where most genes lack introns. In eukaryotic
genes, nucleotide sequence changes within introns are
usually neutral; that is, they do not affect the activity of
the gene product or the phenotype of the organism.
Thus, in the case of eukaryotic genes with introns, there
may be no correlation between gene size and number of
alleles producing altered phenotypes.
12.4 Why was it necessary to modify Beadle and Tatum’s one
gene–one enzyme concept of the gene to one gene–one
polypeptide?
ANS: Several enzymes were shown to contain two or more dif-
ferent polypeptides, and these polypeptides were some-
times controlled by genes that mapped to different
chromosomes. Thus, the mutations clearly were not in
the same gene.
12.5 (a) Why is the genetic code a triplet code instead of a
singlet or doublet code? (b) How many different amino
acids are specied by the genetic code? (c) How many
different amino acid sequences are possible in a polypep-
tide 146 amino acids long?
ANS: (a) Singlet and doublet codes provide a maximum of
4 and (4)
2
or 16 codons, respectively. Thus, neither code
would be able to specify all 20 amino acids. (b) 20.
(c) (20)
146
.
12.6 What types of experimental evidence were used to deci-
pher the genetic code?
ANS: Synthetic RNA molecules (polyuridylic acid molecules)
containing only the base uracil were prepared. When
these synthetic molecules were used to activate in vitro
protein synthesis systems, small polypeptide containing
only the amino acid phenylalanine (polyphenylalanine
molecules) was synthesized. Codons composed only of
uracil were thus shown to specify phenylalanine. Similar
experiments were carried out using synthetic RNA mol-
ecules with different base compositions. Later, in vitro
systems activated with synthetic RNA molecules with
known repeating base sequences were developed. Ulti-
mately, in vitro systems in which specic aminoacyl-
tRNAs where shown to bind to ribosomes activated with
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 45 8/14/2015 6:42:55 PM

46-WC Answers to All Questions and Problems
specic mini-mRNAs, which were trinucleotides of
known base sequence, were developed and used in codon
identication.
12.7 In what sense and to what extent is the genetic code
(a) degenerate, (b) ordered, and (c) universal?
ANS: (a) The genetic code is degenerate in that all but 2 of the
20 amino acids are specied by two or more codons.
Some amino acids are specied by six different codons.
The degeneracy occurs largely at the third or 3′ base of
the codons. “Partial degeneracy” occurs where the third
base of the codon may be either of the two purines or
either of the two pyrimidines and the codon still species
the same amino acid. “Complete degeneracy” occurs
where the third base of the codon may be any one of the
four bases and the codon still species the same amino
acid. (b) The code is ordered in the sense that related
codons (codons that differ by a single base change) spec-
ify chemically similar amino acids. For example, the
codons CUU, AUU, and GUU specify the structurally
related amino acids leucine, isoleucine, and valine,
respectively. (c) The code appears to be almost com-
pletely universal. Known exceptions to universality
include strains carrying suppressor mutations that alter
the reading of certain codons (with low efciencies in
most cases) and the use of UGA as a tryptophan codon in
yeast and human mitochondria.
12.8 The thymine analog 5-bromouracil is a chemical muta-
gen that induces single base-pair substitutions in DNA
called transitions (substitutions of one purine for another
purine and one pyrimidine for another pyrimidine).
Using the known nature of the genetic code (Table 12.1),
which of the following amino acid substitutions should
you expect to be induced by 5-bromouracil with the
highest frequency:
(a) Met → Val; (b) Met → Leu; (c) Lys → Thr; (d) Lys →
Gln; (e) Pro → Arg; or (f) Pro → Gln?
Why?
ANS: (a) Met → Val. This substitution occurs as a result of a
transition. All other amino acid substitutions listed would
require transversions.
12.9 Using the information given in Problem 12.8, would you
expect 5-bromouracil to induce a higher frequency of
His → Arg or His → Pro substitutions? Why?
ANS: Expect a higher frequency of His → Arg substitutions.
His → Arg results from a transition; His → Pro would
require a transversion (not induced by 5-bromouracil).
12.10 What is the minimum number of tRNAs required to rec-
ognize the six codons specifying the amino acid leucine?
ANS: Because of wobble (Table 12.2), one tRNA can recognize
both UUA and UUG codons for leucine. However, it
takes two more tRNAs to recognize CUU, CUC, CUA,
and CUG. Therefore, a minimum of three tRNAs are
required to recognize the six codons for leucine.
12.11 Characterize ribosomes in general as to size, location,
function, and macromolecular composition.
ANS: Ribosomes are from 10 to 20 nm in diameter. They are
located primarily in the cytoplasm of cells. In bacteria,
they are largely free in the cytoplasm. In eukaryotes,
many of the ribosomes are attached to the endoplasmic
reticulum in the cytoplasm. Ribosomes are complex
structures composed of over 50 different polypeptides
and three to ve different RNA molecules. In both pro-
karyotes and eukaryotes, ribosomes are the site of
translation.
12.12 (a) Where in the cells of higher organisms do ribosomes
originate? (b) Where in the cells are ribosomes most
active in protein synthesis?
ANS: (a) The nucleus, specically the nucleoli. (b) The cytoplasm.
12.13 Identify three different types of RNA that are involved in
translation and list the characteristics and functions of
each.
ANS: Messenger RNA (mRNA) molecules carry genetic
information from the chromosomes (where the informa-
tion is stored) to the ribosomes in the cytoplasm (where
the information is expressed during protein synthesis).
The linear sequence of triplet codons in an mRNA
molecule species the linear sequence of amino acids
in the polypeptide(s) produced during translation of
that mRNA. Transfer RNA (tRNA) molecules are
small (about 80 nucleotides long) molecules that carry
amino acids to the ribosomes and provide the codon-
recognition specicity during translation. Ribosomal
RNA (rRNA) molecules provide part of the structure
and function of ribosomes; they represent an impor-
tant part of the machinery required for the synthesis of
polypeptides.
12.14. (a) How is messenger RNA related to polysome forma-
tion? (b) How does rRNA differ from mRNA and tRNA
in specicity? (c) How does the tRNA molecule differ
from that of DNA and mRNA in size and helical
arrangement?
ANS: (a) Polysomes are formed when two or more ribosomes
are simultaneously translating the same mRNA mole-
cule. Ribosomes are usually spaced about 90 nucleotides
apart on an mRNA molecule. Thus, polysome size is
determined by mRNA size. (b) A ribosome, which con-
tains rRNA molecules, can participate in the synthesis of
any polypeptide specied by the ribosome-associated
mRNA. In that sense, rRNA is nonspecic. Messenger
RNAs and tRNAs, in contrast, are specic, in directing the
synthesis of a particular polypeptide or set of polypep-
tides (mRNA) or in attaching to a particular amino acid
(tRNA). (c) Transfer RNA molecules are much smaller
(about 80 nucleotides) than DNA or mRNA molecules.
They are single-stranded molecules but have complex
secondary structures because of the base pairing between
different segments of the molecules.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 46 8/14/2015 6:42:55 PM

Answers to All Questions and Problems WC-47
12.15 Outline the process of aminoacyl-tRNA formation.
ANS: A specic aminoacyl-tRNA synthetase catalyzes the forma-
tion of an amino acid-AMP complex from the appropriate
amino acid and ATP (with the release of pyro pho spha te).
The same enzyme then catalyzes the formation of the
aminoacyl-tRNA complex, with the release of AMP. Both
the amino acid-AMP and aminoacyl-tRNA linkages are
high-energy phosphate bonds.
12.16 How is translation (a) initiated and (b) terminated?
ANS: (a) Translation is initiated by a complex reaction involv-
ing mRNA, ribosomes, initiation factors (IF-1, IF-2, and
IF-3), GTP, the initiator codon AUG, and a special
initiator tRNA (rRNA
f
Met
). It also appears to involve a
base-pairing interaction between a base sequence near
the 3′-end of the 16S rRNA and a base sequence in the
“leader sequence” of the mRNA. (b) Translation is
terminated by recognition of one or more of the chain-
termination codons (UAG, UAA, and UGA) by the
appropriate protein release factor (RF-1 or RF-2).
12.17 Of what signicance is the wobble hypothesis?
ANS: Crick’s wobble hypothesis explains how the anticodon of
a given tRNA can base-pair with two or three different
mRNA codons. Crick proposed that the base-pairing
between the 5′ base of the anticodon in tRNA and the 3′
base of the codon in mRNA was less stringent than
normal and thus allowed some “wobble” at this site. As a
result, a single tRNA often recognizes two or three of
the related codons specifying a given amino acid (see
Table 12.2).
12.18 If the average molecular mass of an amino acid in a par-
ticular polypeptide is 100 daltons, about how many
nucleotides will be present in an mRNA coding sequence
specifying this polypeptide, which has a molecular mass
of 27,000 daltons?
ANS: At least 813 nucleotides [ (270 aa 3) 3 nucleotides
for termination codon].
12.19 The bases A, G, U, C, I (inosine) all occur at the 5′ posi-
tions of anticodons in tRNAs.
(a) Which base can pair with three different bases at the
3′ positions of codons in mRNA? (b) What is the mini-
mum number of tRNAs required to recognize all codons
of amino acids specied by codons with complete
degeneracy?
ANS: (a) Inosine. (b) Two.
12.20 Assume that in the year 2025, the rst expedition of
humans to Mars discovers several Martian life forms
thriving in hydrothermal vents that exist below the plan-
et’s surface. Several teams of molecular biologists extract
proteins and nucleic acids from these organisms and
make some momentous discoveries. Their rst discovery
is that the proteins in Martian life forms contain only 14
different amino acids instead of the 20 present in life
forms on Earth. Their second discovery is that the DNA
and RNA in these organisms have only two different
nucleotides instead of the four nucleotides present in liv-
ing organisms on Earth. (a) Assuming that transcription
and translation work similarly in Martians and Earth-
lings, what is the minimum number of nucleotides that
must be present in the Martian codon to specify all the
amino acids in Martians? (b) Assuming that the Martian
code proposed above has translational start-and-stop
signals, would you expect the Martian genetic code to be
degenerate like the genetic code used on Earth?
ANS: (a) Two nucleotides in all combinations of four (2
4
) would
produce 16 codons. Therefore, the minimum number of
nucleotides comprising the Martian genetic code must
be four. (b) Sixteen codons would allow code words for
14 amino acids, one initiation codon, and a translational
termination codon. The Martian genetic code could not
be degenerate.
12.21 What are the basic differences between translation in
prokaryotes and in eukaryotes?
ANS: Translation occurs by very similar mechanisms in pro-
karyotes and eukaryotes; however, there are some differ-
ences. (1) In prokaryotes, the initiation of translation
involves base-pairing between a conserved sequence
(AGGAGG)—the Shine–Dalgarno box—in mRNA and
a complementary sequence near the 3′ end of the 16S
rRNA. In eukaryotes, the initiation complex forms at the
5′ end of the transcript when a cap-binding protein
interacts with the 7-methyl guanosine on the mRNA.
The complex then scans the mRNA processively and ini-
tiates translation (with a few exceptions) at the AUG
closest to the 5′ terminus. (2) In prokaryotes, the amino
group of the initiator methionyl-tRNA
f
Met
is formylated;
in eukaryotes, the amino group of methionyl-tRNA
i
Met
is
not formylated. (3) In prokaryotes, two soluble protein
release factors (RFs) are required for chain termination.
RF-1 terminates polypeptides in response to UAA and
UAG codons; RF-2 terminates chains in response to
UAA and UGA codons. In eukaryotes, one release factor
responds to all three termination codons.
12.22 What is the function of each of the following compo-
nents of the protein-synthesizing apparatus:
(a) Aminoacyl-tRNA synthetase. (b) Release factor 1.
(c) Peptidyl transferase. (d) Initiation factors. (e) Elonga-
tion factor G
ANS: (a) Attachment of an amino acid to the correct tRNA.
(b) Recognition of termination codons UAA and UAG and
release of the nascent polypeptide from the tRNA in the
P site of the ribosome. (c) Formation of a peptide bond
between the amino group of the aminoacyl-tRNA in the
A site and the carboxyl group of the growing polypeptide
on the tRNA in the P site. (d) Formation of the initiation
complex required for translation; all steps leading up to
peptide bond formation. (e) Translocation of the pepti-
dyl-tRNA from the A site on the ribosome to the P site.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 47 8/14/2015 6:42:56 PM

48-WC Answers to All Questions and Problems
12.23 An E. coli gene has been isolated and shown to be 68 nm
long. What is the maximum number of amino acids that
this gene could encode?
ANS: Assuming 0.34 nm per nucleotide pair in B-DNA, a gene
68 nm long would contain 200 nucleotide pairs. Given
the triplet code, this gene would contain 200/3 66.7
triplets, one of which must specify chain termination.
Disregarding the partial triplet, this gene could encode a
maximum of 65 amino acids.
12.24 (a) What is the difference between a nonsense mutation
and a missense mutation? (b) Are nonsense or missense
mutations more frequent in living organisms? (c) Why?
ANS: (a) A nonsense mutation changes a codon specifying an
amino acid to a chain-termination codon, whereas a mis-
sense mutation changes a codon specifying one amino
acid to a codon specifying a different amino acid. (b)
Missense mutations are more frequent. (c) Of the 64
codons, only three specify chain termination. Thus, the
number of possible missense mutations is much larger
than the number of possible nonsense mutations. More-
over, nonsense mutations almost always produce non-
functional gene products. As a result, nonsense mutations
in essential genes are usually lethal in the homozygous
state.
12.25 The human a-globin chain is 141 amino acids long. How
many nucleotides in mRNA are required to encode
human a-globin?
ANS: 426 nucleotides—3 141 423 specifying amino acids
plus three (one codon) specifying chain termination.
12.26 What are the functions of the A, P, and E aminoacyl-
tRNA binding sites on the ribosome?
ANS: The incoming aminoacyl-tRNA enters the A site of
the ribosome, the nascent polypeptide-tRNA occupies
the P site, and the uncharged exiting tRNA occupies the
E site.
12.27 (a) In what ways does the order in the genetic code mini-
mize mutational lethality? (b) Why do base-pair changes
that cause the substitution of a leucine for a valine in the
polypeptide gene product seldom produce a mutant
phenotype?
ANS: (a) Related codons often specify the same or very similar
amino acids. As a result, single base-pair substitutions
frequently result in the synthesis of identical proteins
(degeneracy) or proteins with amino acid substitutions
involving very similar amino acids. (b) Leucine and valine
have very similar structures and chemical properties;
both have nonpolar side groups and fold into essentially
the same three-dimensional structures when present in
polypeptides. Thus, substitutions of leucine for valine or
valine for leucine seldom alter the function of a protein.
12.28 (a) What is the function of the Shine–Dalgarno sequence
in prokaryotic mRNAs? (b) What effect does the dele-
tion of the Shine–Dalgarno sequence from an mRNA
have on its translation?
ANS: (a) The Shine–Dalgarno sequence is a conserved
polypurine tract, consensus AGGAGG, that is located
about seven nucleotides upstream from the AUG initiation
codon in mRNAs of prokaryotes. It is complementary to,
and is believed to base-pair with, a sequence near the 5′
terminus of the 16S ribosomal RNA. (b) Prokaryotic
mRNAs with the Shine– Dalgarno sequence deleted are
either not translated or are translated inefciently.
12.29 (a) In what ways are ribosomes and spliceosomes similar?
(b) In what ways are they different?
ANS: (a) Both ribosomes and spliceosomes play essential roles
in gene expression, and both are complex macromolecu-
lar structures composed of RNA and protein molecules.
(b) Ribosomes are located in the cytoplasm; spliceosomes
in the nucleus. Ribosomes are larger and more complex
than spliceosomes.
12.30 The 5′ terminus of a human mRNA has the following
sequence:
5′-GAAGAGACAAGGTCAUGGCCAUAUGC
UUGUUCCAAUCGUUAGCUGCGCAGGAUC-
GCCCUGGG . . . . . . 3′
When this mRNA is translated, what amino acid sequence
will be specied by this mRNA sequence?
ANS: NH
2
-Met-Ala-Ile-Cys-Leu-Phe-Gln-Ser-Leu-Ala-
Ala-Gln-Asp-Arg-Pro-Gly-COOH.
12.31 A partial (5′ subterminal) nucleotide sequence of a pro-
karyotic mRNA is as follows:
5′-.....AGGAGGCUCGAACAUGUCAAUAUG CUU
GUUCCAAUCGUUAGCUGCGCAGGACCGUCC
CG GA. . . . . . 3′
When this mRNA is translated, what amino acid
sequence will be specied by this portion of the mRNA?
ANS: Met-Ser-lle-Cys-Leu-Phe-Gln-Ser-Leu-Ala-Ala-
Gln-Asp-Arg-Pro-Gly
12.32 The following DNA sequence occurs in the nontem plate
strand of a gene in a bacterium (the promoter sequence
is located to the left but is not shown):
5′-GAATGTCAGAACTGCCATGCTTCATATGAA-
TAGACCTCTAG-3′
(a) What is the ribonucleotide sequence of the mRNA
molecule that is transcribed from this piece of DNA?
(b) What is the amino acid sequence of the polypeptide
encoded by this mRNA? (c) If the nucleotide indicated
↓
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 48 8/14/2015 6:42:56 PM

Answers to All Questions and Problems WC-49
by the arrow undergoes a mutation that changes T to A,
what will be the resulting amino acid sequence following
transcription and translation?
ANS: (a) 5′-GAAUGUCAGAACUGCCAUGCUUCAUAUG
AAUAGACCUCUAG-3′ (b) NH
2
-fMet-Ser-Glu-Leu-
Pro-Cys-Phe-Ile-COOH (c) NH
2
-fMet-Ser-Glu-Leu-
Pro-Cys-Phe-Ile-Arg-Ile-Asp-Leu-COOH.
12.33 Alan Garen extensively studied a particular nonsense
(chain-termination) mutation in the alkaline phospha-
tase gene of E. coli. This mutation resulted in the termi-
nation of the alkaline phosphatase polypeptide chain at a
position where the amino acid tryptophan occurred in
the wild-type polypeptide. Garen induced revertants (in
this case, mutations altering the same codon) of this
mutant with chemical mutagens that induced single
base-pair substitutions and sequenced the polypeptides
in the revertants. Seven different types of revertants
were found, each with a different amino acid at the tryp-
tophan position of the wild-type polypeptide (termina-
tion position of the mutant polypeptide fragment). The
amino acids present at this position in the various rever-
tants included tryptophan, serine, tyrosine, leucine, glu-
tamic acid, glutamine, and lysine. Did the nonsense
mutation studied by Garen contain a UAG, a UAA, or a
UGA nonsense mutation? Explain the basis of your
deduction.
ANS: (UAG). This is the only nonsense codon that is related to
tryptophan, serine, tyrosine, leucine, glutamic acid, glu-
tamine, and lysine codons by a single base-pair substitu-
tion in each case.
12.34 The following DNA sequence occurs in a bacterium
(the promoter sequence is located to the left but is not
shown).
↓
5′-CAATCATGGACTGCCATGCTTCATATGAATAGTTGACAT-3′
3′-GTTAGTACCTGACGGTACGAAGTATACTTATCAACTGTA-5′
(a) What is the ribonucleotide sequence of the mRNA
molecule that is transcribed from the template strand of
this piece of DNA? Assume that both translational start
and termination codons are present.
(b) What is the amino acid sequence of the polypeptide
encoded by this mRNA?
(c) If the nucleotide indicated by the arrow undergoes a
mutation that causes this C:G base pair to be deleted,
what will be the polypeptide encoded by the mutant
gene?
ANS: (a) 5′-CAAUCAUGGACUGCCAUGCUUCAUAUG
AAUAGUUGACAU-3′ (b) NH2-fMet-Asp-Cys-His-Ala-
Ser-Tyr-Glu-COOH (c) NH2-fMet-Asp-Cys -Met-Leu-
His -Met-Asn-Ser- COOH.
Chapter 13
13.1 Identify the following point mutations represented in
DNA and in RNA as (1) transitions, (2) transversions, or
(3) reading frameshifts. (a) A to G; (b) C to T; (c) C to G;
(d) T to A; (e) UAU ACC UAU to UAU AAC CUA;
(f) UUG CUA AUA to UUG CUG AUA.
ANS: (a) Transition, (b) transition, (c) transversion, (d) trans-
version, (e) frameshift, (f) transition.
13.2 Of all possible missense mutations that can occur in a
segment of DNA encoding the amino acid tryptophan,
what is the ratio of transversions to transitions if
all single base-pair substitutions occur at the same
frequency?
ANS: 6:1. UGG transitions: UGA (nonsense), UAG (nonsense),
CGG (Arg). UGG transversions: UGC (Cys), UGU
(Cys), UCG (Ser), UUG (Leu), AGG (Arg), GGG (Gly).
13.3 Both lethal and visible mutations are expected to occur
in fruit ies that are subjected to irradiation. Outline a
method for detecting (a) X-linked lethals and (b) X-linked
visible mutations in irradiated Drosophila.
ANS: (a) ClB method, (b) attached X method (see Chapter 6).
13.4 H. J. Muller used the ClB technique to identify many
radiation-induced recessive lethal mutations on Dro-
sophila’s X chromosome, which is now known to con-
tain more than a thousand genes. These mutations
could be propagated in stock cultures by keeping them
in heterozygous condition with the ClB chromosome.
Would you expect all these lethal mutations to be
alleles of one essential X-linked gene, or to be alleles
of different essential X-linked genes? Why couldn’t
H. J. Muller determine the answer to this question
experimentally?
ANS: The radiation-induced recessive lethal mutations that
Muller recovered in his experiments were likely
scattered across the entire X chromosome. Thus, they
likely affected different genes. However, Muller could
not study this issue because he could not carry out a
complementation test to determine if any of the lethal
mutations were alleles of the same gene. The reason is
that to perform the complementation test, Muller would
have had to cross females that carried a particular lethal
mutation balanced with the ClB chromosome to males
that carried a different lethal mutation. Because such
males are not viable, the required cross cannot be
performed.
13.5 Published spontaneous mutation rates for humans are
generally higher than those for bacteria. Does this indi-
cate that individual genes of humans mutate more fre-
quently than those of bacteria? Explain.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 49 8/14/2015 6:42:56 PM

50-WC Answers to All Questions and Problems
ANS: Probably not. A human is larger than a bacterium, with
more cells and a longer life span. If mutation frequencies
are calculated in terms of cell generations, the rates for
human cells and bacterial cells are similar.
13.6 A precancerous condition (intestinal polyposis) in a par-
ticular human family group is caused by a single domi-
nant gene. Among the descendants of one woman who
died with cancer of the colon, 10 people have died with
the same type of cancer and 6 now have intestinal pol-
yposis. All other branches of the large kindred have been
carefully examined, and no cases have been found. Sug-
gest an explanation for the origin of the defective gene.
ANS: A dominant mutation presumably occurred in the woman
in whom the condition was rst known.
13.7 Juvenile muscular dystrophy in humans depends on an
X-linked recessive gene. In an intensive study, 33 cases
were found in a population of some 800,000 people. The
investigators were condent that they had found all cases
that were well enough advanced to be detected at the
time the study was made. The symptoms of the disease
were expressed only in males. Most of those with the dis-
ease died at an early age, and none lived beyond 21 years
of age. Usually, only one case was detected in a family,
but sometimes two or three cases occurred in the same
family. Suggest an explanation for the sporadic occur-
rence of the disease and the tendency for the gene to
persist in the population.
ANS: The X-linked gene is carried by mothers, and the disease
is expressed in half of their sons. Such a disease is dif-
cult to follow in pedigree studies because of the recessive
nature of the gene, the tendency for the expression to
skip generations in a family line, and the loss of the males
who carry the gene. One explanation for the sporadic
occurrence and tendency for the gene to persist is that,
by mutation, new defective genes are constantly being
added to the load already present in the population.
13.8 Products resulting from somatic mutations, such as the
navel orange and the Delicious apple, have become
widespread in citrus groves and apple orchards. However,
traits resulting from somatic mutations are seldom main-
tained in animals. Why?
ANS: Plants can be propagated vegetatively, but no such meth-
ods are available for widespread use in animals.
13.9 If a single short-legged sheep should occur in a ock,
suggest experiments to determine whether the short legs
are the result of a mutation or an environmental effect. If
due to a mutation, how can one determine whether the
mutation is dominant or recessive?
ANS: The sheep with short legs could be mated to unrelated
animals with long legs. If the trait is expressed in the rst
generation, it could be presumed to be inherited and to
depend on a dominant gene. On the other hand, if it does
not appear in the rst generation, F
1
sheep could be
crossed back to the short-legged parent. If the trait is
expressed in one-half of the backcross progeny, it is prob-
ably inherited as a simple recessive. If two short-legged
sheep of different sex could be obtained, they could be
mated repeatedly to test the hypothesis of dominance. In
the event that the trait is not transmitted to the progeny
that result from these matings, it might be considered to
be environmental or dependent on some complex genetic
mechanism that could not be identied by the simple test
used in the experiments.
13.10 How might enzymes such as DNA polymerase be in volved
in the mode of action of both mutator and antimutator
genes (mutant genes that increase and decrease, respec-
tively, mutation rates)?
ANS: Enzymes may discriminate among the different nucleo-
tides that are being incorporated. Mutator enzymes may
utilize a higher proportion of incorrect nucleotides,
whereas antimutator enzymes may select fewer incorrect
bases in DNA replication. In the case of the phage T4
DNA polymerase, the relative efciencies of polymeriza-
tion and proofreading by the polymerase’s 3′ → 5′ exo-
nuclease activity play key roles in determining the
mutation rate.
13.11 How could spontaneous mutation rates be optimized by
natural selection?
ANS: If both mutators and antimutators operate in the same
living system, an optimum mutation rate for a particular
organism in a given environment may result from natu-
ral selection.
13.12 A mutator gene Dt in maize increases the rate at which
the gene for colorless aleurone (a) mutates to the domi-
nant allele (A), which yields colored aleurone. When
reciprocal crosses were made (i.e., seed parent dt/dt, a/a
Dt/Dt, a/a and seed parent Dt/Dt, a/a dt/dt, a/a), the
cross with Dt/Dt seed parents produced three times as
many dots per kernel as the reciprocal cross. Explain
these results.
ANS: Dt is a mutator gene that induces somatic mutations in
developing kernels.
13.13 The deciency Df(1)w
rJ1
removes 16 contiguous bands
from a region near the left end of the Drosophila X chro-
mosome. Females homozygous for this deciency die.
However, females heterozygous for it and a ClB chromo-
some are viable and fertile. If such females are mated to
males that carry wild-type X and Y chromosomes, what
kinds of progeny will appear and in what proportions?
ANS: The cross is Df(1)w
rJ1
/ClB females /Y (wild-type)
males. The genotypes of the daughters are Df(1)w
rJ1
/
(phenotypically wild-type) and ClB/ (bar-eyed). These
two classes of daughters will occur in equal proportions.
The sons inherit a Y chromosome and either the Df(1)
w
rJ1
or ClB X chromosomes, both of which act as reces-
sive lethals. Thus, no sons will appear in the progeny.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 50 8/14/2015 6:42:56 PM
Answers to All Questions and Problems WC-51
13.14 In Drosophila, the Y chromosome Y·w
has a small piece
of the X chromosome translocated to it; this piece con-
tains the wild-type alleles of all the genes missing in
Df(1)w
rJ1
mentioned in Problem 13.13. If males carrying
Y·w
and a wild-type X chromosome are crossed to Df(1)
w
rJ1
/ClB females, what kinds of progeny will appear, and
in what proportions? How would your answer change if
the wild-type X chromosome in the males carried a radi-
ation-induced recessive lethal mutation located within
the region that is missing in Df(1)w
rJ1
? How could these
unusual chromosomes be used to devise a scheme that
would allow you to carry out complementation tests
between two independently induced recessive lethal
mutations that map within this region?
ANS: In the cross Df(1)w
rJ1
/ClB females /Y·w
(wild-type)
males, the daughters will be either Df(1)w
rJ1
/ (pheno-
typically wild-type) or ClB/ (bar-eyed); these two types
of daughters will appear in equal proportions. The sons
from the cross will be either Df(1)w
rJ1
/ Y·w
(viable and
phenotypically wild-type because the small piece of the
X chromosome translocated to the Y chromosome con-
tains the genes that are missing in the deciency Df(1)
w
rJ1
) or ClB/ Y·w
. This latter genotype will be viable if
the lethal mutation in the ClB chromosome resides in the
region dened by the deciency Df(1)w
rJ1
. In that case,
bar-eyed males would appear and be as frequent as wild-
type males. If the lethal mutation in the ClB chromo-
some resides outside the region dened by Df(1)w
rJ1
,
then no bar-eyed males will appear among the progeny.
If the males in the cross carry a radiation-induced lethal
(ril) mutation in the region dened by Df(1)w
rJ1
—that is,
if the cross is Df(1)w
rJ1
/ClB females ril/Y·w
males
(viable because the small piece of the X chromosome
carried by the Y chromosome includes the wild-type
allele of ril)—there will not be any wild-type daughters
(genotype Df(1)w
rJ1
/ril) among the progeny. However,
bar-eyed daughters (genotypically ClB/ril should appear
unless the lethal mutation in the ClB chromosome is
allelic to ril.
An induced lethal mutation that lies within the region
dened by Df(1)w
rJ1
could be tested for complementation
with another such lethal mutation. Let’s call the rst
mutation lethal-1 and the second mutation lethal-2. The
required cross for the complementation test is ClB/lethal-1
females lethal-2/Y·w
males. This cross is possible
because the small piece of the X chromosome carried by
the Y·w
chromosome contains the wild-type allele of
lethal-2; thus, the lethal-2/Y·w
males are viable. Among
the progeny, we would look for daughters with the geno-
type lethal-1/lethal-2, which, because they lack the ClB
chromosome, will have normal (non-bar) eyes. If these
females appear, we know that lethal-1 and lethal-2 comple-
ment one another; that is, they are mutations in different
genes. If these females do not appear, we can conclude that
lethal-1 and lethal-2 are alleles of the same gene.
13.15 If CTT is a DNA triplet (transcribed strand of DNA)
specifying glutamic acid, what DNA and mRNA base
triplet alterations could account for valine and lysine in
position 6 of the b-globin chain?
ANS:
Amino Acid
Glumatic acid
Valine
GUA
Lysine
AAA
AAA
TTT
CAT
GTA
CTT
Transcribed strand
Mutation
Mutation
GAA
mRNA DNA
GAA
... .. ..
... .. ..
... .. ..
13.16 The bacteriophage T4 genome contains about 50
percent A:T base pairs and 50 percent G:C base pairs. The
base analog 2-aminopurine induces A:T → G:C and G:C
→ A:T base-pair substitutions by undergoing tautomeric
shifts. Hydroxylamine is a mutagenic chemical that reacts
specically with cytosine and induces only G:C → A:T
substitutions. If a large number of independent muta-
tions were produced in bacteriophage T4 by treatment
with 2-aminopurine, what percentage of these mutations
should you expect to be induced to mutate back to the
wild-type genotype by treatment with hydroxylamine?
ANS: About half of the induced mutations would be expected
to mutate back to the wild-type genotype.
13.17 Assuming that the b-globin chain and the a-globin chain
shared a common ancestor, what mechanisms might
explain the differences that now exist in these two chains?
What changes in DNA and mRNA codons would
account for the differences that have resulted in unlike
amino acids at corresponding positions?
ANS: Mutations: transitions, transversions, and frameshifts.
13.18 In a given strain of bacteria, all of the cells are usually
killed when a specic concentration of streptomycin is
present in the medium. Mutations that confer resistance
to streptomycin occur. The streptomycin-resistant
mutants are of two types: some can live with or without
streptomycin; others cannot survive unless this drug is
present in the medium. Given a streptomycin-sensitive
strain of this species, outline an experimental procedure
by which streptomycin-resistant strains of the two types
could be established.
ANS: Irradiate the nonresistant strain and plate the irradiated
organisms on a medium containing streptomycin. Those
that survive and produce colonies are resistant. They
could then be replicated to a medium without strepto-
mycin. Those that survive would be of the rst type;
those that can live with streptomycin but not without it
would be the second type.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 51 8/14/2015 6:42:56 PM

52-WC Answers to All Questions and Problems
13.19 One stock of fruit ies was treated with 1000 roentgens
(r) of X-rays. The X-ray treatment increased the muta-
tion rate of a particular gene by 2 percent. What percent-
age increases in the mutation rate of this gene would be
expected if this stock of ies was treated with X-ray doses
of 1500 r, 2000 r, and 3000 r?
ANS: 3%; 4%; 6%.
13.20 Why does the frequency of chromosome breaks induced
by X-rays vary with the total dosage and not with the
rate at which it is delivered?
ANS: Each quantum of energy from the X-rays that is absorbed
in a cell has a certain probability of hitting and breaking
a chromosome. Hence, the greater the number of quanta
of energy or dosage, the more likely the breaks are to
occur. The rate at which this dosage is delivered does not
change the probability of each quantum inducing a
break.
13.21 A reactor overheats and produces radioactive tritium
(H
3
), radioactive iodine (I
131
), and radioactive xenon
(Xn
133
). Why should we be more concerned about radio-
active iodine than the other two radioactive isotopes?
ANS: Radioactive iodine is concentrated by living organisms
and food chains.
13.22 One person was in an accident and received 50 roentgens
(r) of X-rays at one time. Another person received 5 r in
each of 20 treatments. Assuming no intensity effect, what
proportionate number of mutations would be expected
in each person?
ANS: The person receiving a total of 100 r would be expected
to have twice as many mutations as the one receiving
50 r.
13.23 A cross was performed in Neurospora crassa between a
strain of mating type A and genotype x
m
z and a strain
of mating type a and genotype x m z
. Genes x, m, and z
are closely linked and are present in the order x–m–z on
the chromosome. An ascus produced from this cross
contained two copies (“identical twins”) of each of the
four products of meiosis. If the genotypes of the four
products of meiosis showed that gene conversion had
occurred at the m locus and that reciprocal recombina-
tion had occurred at the x and z loci, what might the
genotypes of the four products look like? In the paren-
theses below, write the genotypes of the four haploid
products of meiosis in an ascus showing gene conversion
at the m locus and reciprocal recombination of the ank-
ing markers (at the x and z loci).
Ascus Spore Pairs
1–2 3–4 5–6 7–8
( ) ( ) ( ) ( )
ANS: ( x
m
z) (x
m
z
) (x m
z) (x m z
) or equivalent.
13.24 How does nitrous acid induce mutations? What specic
end results might be expected in DNA and mRNA from
the treatment of viruses with nitrous acid?
ANS: Nitrous acid brings about a substitution of an OH group
for an NH
2
group in those bases (A, C, and G) having
NH
2
side groups. In so doing, adenine is converted into
hypoxanthine, which base-pairs with cytosine, and cyto-
sine is converted into uracil, which base-pairs with ade-
nine. The net effects are GC ↔ AT base-pair substitutions
(see Figure 13.16).
13.25 Are mutational changes induced by nitrous acid more
likely to be transitions or transversions?
ANS: Transitions.
13.26 You are screening three new pesticides for potential
mutagenicity by using the Ames test. Two his
strains
resulting from either a frameshift or a transition muta-
tion were used and produced the following results (num-
ber of revertant colonies):
Strain 1
Transition
Mutant
Control
(no chemical)
Transion
Mutant
Chemical
Transion Mutant
Chemical
Rat liver
Enzymes
Pesticide #1 21 180 19
Pesticide #2 18 19 17
Pesticide #3 25 265 270
Strain 2
Frameshift
Mutant
Control
(no chemical)
Frameshift
Mutant
Chemical
Frameshift
Mutant
Chemical Rat
liver Enzymes
Pesticide #1 5 4 5
Pesticide #2 7 5 93
Pesticide #3 6 9 7
What type of mutations, if any, do the three pesticides
induce?
ANS: P #1—Causes transition mutation. Liver enzymes convert
it into nonmutagen. Does not cause frameshift mutations.
P #2—Does not cause transition mutations. Liver enzymes
convert it into a frameshift mutagen. P #3—Causes
transition mutations. Liver enzymes have no effect on
mutagenicity. Does not cause frameshift mutations.
13.27 How does the action and mutagenic effect of 5-bromo-
uracil differ from that of nitrous acid?
ANS: Nitrous acid acts as a mutagen on either replicating or
nonreplicating DNA and produces transitions from A to
G or C to T, whereas 5-bromouracil does not affect non-
replicating DNA but acts during the replication process
causing GC ↔ AT transitions. 5-Bromouracil must be
incorporated into DNA during the replication process in
order to induce mispairing of bases and thus mutations.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 52 8/14/2015 6:42:57 PM

Answers to All Questions and Problems WC-53
13.28 Sydney Brenner and A. O. W. Stretton found that non-
sense mutations did not terminate polypeptide synthesis
in the rII gene of the bacteriophage T4 when these
mutations were located within a DNA sequence interval
in which a single nucleotide insertion had been made on
one end and a single nucleotide deletion had been made
on the other. How can this nding be explained?
ANS: The reading frame will be shifted between the two
frameshift mutations. This shift in reading frame does
not read the normal nonsense codon and termination
does not occur.
13.29 Seymour Benzer and Ernst Freese compared spontane-
ous and 5-bromouracil-induced mutants in the rII gene
of the bacteriophage T4; the mutagen increased the
mutation rate (rII
→ rII) several hundred times above
the spontaneous mutation rate. Almost all (98 percent) of
the 5-bromouracil-induced mutants could be induced to
revert to wild-type (rII → rII
) by 5-bromouracil treat-
ment, but only 14 percent of the spontaneous mutants
could be induced to revert to wild-type by this treatment.
Discuss the reason for this result.
ANS: 5-BU causes GC ↔ AT transitions. 5-BU can, therefore,
revert almost all of the mutations that it induces by
enhancing the transition event that is the reverse of the
one that produced the mutation. In contrast, the sponta-
neous mutations will include transversions, frameshifts,
deletions, and other types of mutations, including
transitions. Only the spontaneous transitions will show
enhanced reversion after treatment with 5-BU.
13.30 How do acridine-induced changes in DNA result in
inactive proteins?
ANS: Mutations induced by acridine dyes are primarily inser-
tions or deletions of single base-pairs. Such mutations
alter the reading frame (the in-phase triplets specifying
mRNA codons) for that portion of the gene distal (rela-
tive to the direction of transcription and translation) to
the mutation (see Figure 13.7b). This would be expected
to totally change the amino acid sequences of polypep-
tides distal to the mutation site and produce inactive
polypeptides. In addition, such frameshift mutations fre-
quently produce in-frame termination codons that result
in truncated proteins.
Use the known codon-amino acid assignments (Table
12.1) to work the following problems.
13.31 Mutations in the genes encoding the - and -subunits
of hemoglobin lead to blood diseases such as thalassemia
and sickle-cell anemia. You have found a family in China
in which some members suffer from a new genetic form
of anemia. The DNA sequences at the 5′ end of the non-
template strand of the normal and mutant DNA encod-
ing the subunit of hemoglobin are as follows:
Normal 5-ACGTTATGCCGTACTGCCAGCTAAC
TGCTAAAGAACAATTA……-3
Mutant 5-ACGTTATGCCCGTACTGCCAGCTAA
CTGCTAAAGAACAATTA….-3
(a) What type of mutation is present in the mutant hemo-
globin gene? (b) What are the codons in the translated
portion of the mRNA transcribed from the normal and
mutant genes? (c) What are the amino acid sequences of
the normal and mutant polypeptides?
ANS: (a) Frameshift due to the insertion of C at the 9th, 10th, or
11th nucleotide from the 5′ end. (b) Normal: 5′-AUGCC-
GUACUGCCAGCUAACUGCUAAAGAACAAUUA-3′.
Mutant: 5′-AUGCCCGUACUGCCAGCUAACUGC-
UAAAGAACAAUUA-3′. (c) Normal: NH
2
-Met-Pro-Tyr-
Cys-Gln-Leu-Thr-Ala-Lys-Glu-Gln-Leu. Mutant: NH
2
-
Met-Pro-Val-Leu-Pro-Ala-Asn-Cys.
13.32 Bacteriophage MS2 carries its genetic information in
RNA. Its chromosome is analogous to a polygenic mol-
ecule of mRNA in organisms that store their genetic
information in DNA. The MS2 minichromosome
encodes four polypeptides (i.e., it has four genes). One of
these four genes encodes the MS2 coat protein, a poly-
peptide of 129 amino acids long. The entire nucleotide
sequence in the RNA of MS2 is known. Codon 112 of
the coat protein gene is CUA, which species the amino
acid leucine. If you were to treat a replicating population
of bacteriophage MS2 with the mutagen 5-bromouracil,
what amino acid substitutions would you expect to be
induced at position 112 of the MS2 coat protein (i.e.,
Leu → other amino acid)? (Note: Bacteriophage MS2
RNA replicates using a complementary strand of RNA
and base-pairing as DNA.)
ANS: Proline and serine.
13.33 Would the different amino acid substitutions induced by
5-bromouracil at position 112 of the coat polypeptide
that you indicated in Problem 13.32 be expected to occur
with equal frequency? If so, why? If not, why not? Which
one(s), if any, would occur more frequently?
ANS: No. Leucine → proline would occur more frequently.
Leu (CUA) — 5-BU → Pro (CCA) occurs by a single
base-pair transition, whereas Leu (CUA) — 5-BU → Ser
(UCA) requires two base-pair transitions. Recall that
5-bromouracil (5-BU) induces only transitions (see
Figure 13.15).
13.34 Would such mutations occur if a nonreplicating suspen-
sion of MS2 phage was treated with 5-bromouracil?
ANS: No. 5-Bromouracil is mutagenic only to replicating
nucleic acids.
13.35 Recall that nitrous acid deaminates adenine, cytosine, and
guanine (adenine → hypoxanthine, which base-pairs with
cytosine; cytosine → uracil, which base-pairs with adenine;
and guanine → xanthine, which base-pairs with cytosine).
Would you expect nitrous acid to induce any mutations
that result in the substitution of another amino acid for a
glycine residue in a wild-type polypeptide (i.e., glycine →
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 53 8/14/2015 6:42:57 PM
54-WC Answers to All Questions and Problems
another amino acid) if the mutagenesis were carried out
on a suspension of mature (nonreplicating) T4 bacterio-
phage? (Note: After the mutagenic treatment of the phage
suspension, the nitrous acid is removed. The treated phage
is then allowed to infect E. coli cells to express any induced
mutations.) If so, by what mechanism? If not, why not?
ANS: Yes:
DNA:
mRNA:
Polypeptide:
or
GGX
GGX
HNO
2
AGX
Gly Ser or Arg
(depending on X)
CCX'
GGX
UCX'
DNA:
mRNA:
Polypeptide:
or
GGX
GGX
HNO
2
GAX
Gly Asp or Glu
(depending on X)
CCX'
GGX
CUX'
DNA:
mRNA:
Polypeptide:
GGX
GGX
HNO
2
AAX
Gly Asn or Lys
(depending on X)
CCX'
GGX
UUX'
Note: The X at the third position in each codon in mRNA
and in each triplet of base pairs in DNA refers to the fact
that there is complete degeneracy at the third base in the
glycine codon. Any base may be present in the codon,
and it will still specify glycine.
13.36 Keeping in mind the known nature of the genetic code,
the information given about phage MS2 in Problem
13.32, and the information you have learned about
nitrous acid in Problem 13.35, would you expect
nitrous acid to induce any mutations that would result
in amino acid substitutions of the type glycine →
another amino acid if the mutagenesis were carried out
on a suspension of mature (nonreplicating) MS2 bacte-
riophage? If so, by what mechanism? If not, why not?
ANS: No. The glycine codon is GGX, where X can be any one
of the four bases. Because of this complete degeneracy
at the third position of the glycine codon, changing X
to any other base will have no effect (i.e., the codon will
still specify glycine). Nitrous acid deaminates guanine
(G) to xanthine, but xanthine still base-pairs with cyto-
sine. Thus, guanine is not a target for mutagenesis by
nitrous acid.
13.37 Would you expect nitrous acid to induce a higher fre-
quency of Tyr → Ser or Tyr → Cys substitutions? Why?
ANS: Tyr → Cys substitutions; Tyr to Cys requires a transition,
which is induced by nitrous acid. Tyr to Ser would
require a transversion, and nitrous acid is not expected to
induce transversions.
13.38 Which of the following amino acid substitutions should
you expect to be induced by 5-bromouracil with the
highest frequency? (a) Met → Leu; (b) Met → Thr;
(c) Lys → Thr; (d) Lys → Gln; (e) Pro → Arg; or (f) Pro
→ Gln? Why?
ANS: (b) Met → Thr. 5-Bromouracil induces transitions, not
transversions. All other changes listed require transversions.
13.39 The wild-type sequence of part of a protein is
NH
2
-Trp-Trp-Trp-Met-Arg-Glu-Trp-Thr-Met
Each mutant in the following table differs from wild-
type by a single point mutation. Using this information,
determine the mRNA sequence coding for the wild-type
polypeptide. If there is more than one possible nucleo-
tide, list all possibilities.
Mutant Amino Acid Sequence of Polypeptide
1 Trp-Trp-Trp Met
2 Trp-Trp-Trp-Met-Arg-Asp-Trp-Thr-Met
3 Trp-Trp-Trp-Met-Arg-Lys-Trp-Thr-Met
4 Trp-Trp-Trp-Met-Arg-Glu-Trp-Met-Met
ANS: 5′-UGG-UGG-UGG-AUG-CGA(or AGA)-GAA(or
GAG)-UGG-AUG-3′
13.40 Acridine dyes such as proavin are known to induce
primarily single base-pair additions and deletions. Sup-
pose that the wild-type nucleotide sequence in the
mRNA produced from a gene is
5′-AUGCCCUUUGGGAAAGGGUUUCCCUAA-3′
Also, assume that a mutation is induced within this gene by
proavin, and, subsequently, a revertant of this mutation is
similarly induced with proavin and shown to result from
a second-site suppressor mutation within the same gene. If
the amino acid sequence of the polypeptide encoded by
this gene in the revertant (double mutant) strain is
NH
2
-Met-Pro-Phe-Gly-Glu-Arg-Phe-Pro-COOH
what would be the most likely nucleotide sequence in the
mRNA of this gene in the revertant (double mutant)?
ANS: 5′-AUGCCCUUUGGGGAAAGGUUUCCCUAA-3′
13.41 Eight independently isolated mutants of E. coli, all of
which are unable to grow in the absence of histidine (his
−
),
were examined in all possible cis and trans heterozygotes
(partial diploids). All of the cis heterozygotes were able to
grow in the absence of histidine. The trans heterozygotes
yielded two different responses: some of them grew in the
absence of histidine; others did not. The experimental
results, using “” to indicate growth and “0” to indicate
no growth, are given in the accompanying table. How
many genes are dened by these eight mutations? Which
mutant strains carry mutations in the same gene(s)?
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 54 8/14/2015 6:42:57 PM
Answers to All Questions and Problems WC-55
Growth of Trans Heterozygotes (without Histidine)
Mutant 1 2 3 4 5 6 7 8
8 0 0 0 0 0 0 1 0
7
0
6 0 0 0 0 0 0
5 0 0 0 0 0
4 0 0 0 0
3 0 0 0
2 0 0
1 0
ANS: Two genes; mutations 1, 2, 3, 4, 5, 6, and 8 are in one
gene; mutation 7 is in a second gene.
13.42 Assume that the mutants described in Problem 13.41
yielded the following results. How many genes would
they have dened? Which mutations would have been in
the same gene(s)?
Mutant 1 2 3 4 5 6 7 8
8
0 0
7
0
6
0 0
5
0
4
0 0
3
0
2 0 0
1 0
ANS: Four genes; mutations 1 and 2 in one gene; mutations 3
and 4 in a second gene; mutations 5 and 6 in a third gene;
and mutations 7 and 8 in a fourth gene.
13.43 In Drosophila, white, white cherry, and vermilion are all sex-
linked mutations affecting eye color. All three mutations
are recessive to their wild-type allele(s) for red eyes. A
white-eyed female crossed with a vermilion-eyed male
produces white-eyed male offspring and red-eyed (wild-
type) female offspring. A white-eyed female crossed with
a white cherry-eyed male produces white-eyed sons and
light cherry-eyed daughters. Do these results indicate
whether or not any of the three mutations affecting eye
color are located in the same gene? If so, which
mutations?
ANS: The complementation test for allelism involves placing
mutations pairwise in a common protoplasm in the trans
conguration and determining whether the resulting
trans heterozygotes have wild-type or mutant pheno-
types. If the two mutations are in different genes, the
two mutations will complement each other, because the
wild-type copies of each gene will produce functional
gene products (see Figure 13.21a). However, if the two
mutations are in the same gene, both copies of the gene in
the trans heterozygote will produce defective gene prod-
ucts, resulting in a mutant phenotype (see Figure 13.21b).
When complementation occurs, the trans heterozy-
gote will have the wild-type phenotype. Thus, the
complementation test allows one to determine whether
any two recessive mutations are located in the same gene
or in different genes. Because the mutations of interest
are sex-linked, all the male progeny will have the same
phenotype as the female parent. They are hemizygous,
with one X chromosome obtained from their mother. In
contrast, the female progeny are trans heterozygotes. In
the cross between the white-eyed female and the vermil-
ion-eyed male, the female progeny have red eyes, the
wild-type phenotype. Thus, the white and vermilion
mutations are in different genes, as illustrated in the
following diagram:
X chr
omosome
from parent
trans heterozygote
X chr
omosome
from parent
Complementation yields wild-type phenotype; both v
+
and w
+
gene products are produced in the trans heterozygote.
v
v
+
w
+
v
+
gene product
w
+
gene pr
oduct
w
In the cross between a white-eyed female and a white
cherry-eyed male, the female progeny have light cherry-
colored eyes (a mutant phenotype), not wild-type red
eyes as in the rst cross. Since the trans heterozygote has
a mutant phenotype, the two mutations, white and white
cherry, are in the same gene:
trans heterozygote
No w
+
gene product; therefore, mutant phenotype.
w
ch
No active (w
+
)
gene product
w
X chr
omosome
from parent
X chr
omosome
from parent
13.44 The loz (lethal on Z) mutants of bacteriophage X are con-
ditional lethal mutants that can grow on E. coli strain
Y but cannot grow on E. coli strain Z. The results shown
in the following table were obtained when seven loz
mutants were analyzed for complementation by infect-
ing E. coli strain Z with each possible pair of mutants.
A “” indicates that progeny phage were produced in
the infected cells, and a “0” indicates that no progeny
phage were produced. All possible cis tests were also
done, and all cis heterozygotes produced wild-type yields
of progeny phage.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 55 8/14/2015 6:42:58 PM

56-WC Answers to All Questions and Problems
Mutant 1 2 3 4 5 6 7
7
0
0 0 0
6
0
5
0
0
4 0 0
0
3
0
2 0 0
1 0
Propose three plausible explanations for the apparently
anomalous complementation behavior of loz mutant
number 7. (b) What simple genetic experiments can be
used to distinguish between the three possible explana-
tions? (c) Explain why specic outcomes of the proposed
experiments will distinguish between the three possible
explanations.
ANS: Mutations 1, 2, and 4 do not complement one another
and thus appear to be located in the same gene, as
do mutations 3 and 5. The anomaly is that mutation 7
does not complement mutations 3, 5, and 6, even
though mutation 6 does complement mutations 3 and
5. (a) There are three simple explanations of the seem-
ingly anomalous complementation behavior of muta-
tion 7. (1) It is a deletion spanning all or parts of two
genes. (2) It is a double mutation with defects in two
genes. (3) It is a polar mutation—a nonsense mutation
that interferes with the expression of downstream
genes—in the promoter-proximal gene of a multigenic
transcription unit. (b) Three simple genetic operations
will distinguish between these three possibilities.
(1) Reversion. Plate a large number of mutant 7 phage
on E. coli strain Z and look for wild-type revertants.
(2) Backcross mutant 7 to wild-type phage and test the
mutant progeny for the ability to complement muta-
tions 3 and 6. (3) Introduce F’s carrying tRNA non-
sense suppressor genes into E. coli strain Z and
determine whether any of them suppress the loz7
mutation. (c) If mutation 7 is a deletion, it will not
revert, and, if it is a double mutation, the reversion
rate will probably be below the level of detection in
your experiment. On the other hand, if it is a polar
nonsense mutation, loz
revertants will be obtained. If
mutation 7 is a deletion, no new genotypes will be pro-
duced in the backcross to wild-type. However, if it is a
double mutation, some recombinant single-mutant
progeny will be produced in the backcross to wild-
type, and these single mutations will complement
either mutation 3 or mutation 6. If mutation 7 is a
polar nonsense mutation, it should be suppressed by
one or more of the tRNA suppressor genes introduced
into E. coli strain Z.
Chapter 14
14.1 (a) In what ways is the introduction of recombinant DNA
molecules into host cells similar to mutation? (b) In what
ways is it different?
ANS: (a) Both introduce new genetic variability into the cell. In
both cases, only one gene or a small segment of DNA
representing a small fraction of the total genome is
changed or added to the genome. The vast majority of
the genes of the organism remain the same. (b) The
introduction of recombinant DNA molecules, if they
come from a very different species, is more likely to
result in a novel, functional gene product in the cell, if
the introduced gene (or genes) is capable of being
expressed in the foreign protoplasm. The introduction of
recombinant DNA molecules is more analogous to
duplication mutations (see Chapter 6) than to other
types of mutations.
14.2 Listed in this question are four different single strands of
DNA. Which of these, in their double-stranded form,
would you expect to be cleaved by a restriction
endonuclease?
(a) ACTCCAGAATTCACTCCG
(b) GCCTCATTCGAAGCCTGA
(c) CTCGCCAATTGACTCGTC
(d) ACTCCACTCCCGACTCCA
ANS: Restriction endonucleases cleave at palindromes in dou-
ble-stranded DNA. A palindrome (indicated in bold-
faced letters) can be found in all the double-stranded
sequences except d. Therefore, sequence d would not be
cleaved by a restriction endonuclease, whereas a, b,
and c could be cleaved by the appropriate enzyme.
(a)
ACTCCAGA ATTC ACTCCG
TGCGGTCTTA AGTGAGGC
(b)
GCCTCATTCGA AGCCTGA
CGGAGTAAGCTTCGGACT
(c)
CTCGCCA ATTGACTCGTC
GAGCGGTTAACTGAGCAG
(d)
ACTCCACTCCCGACTCCA
TGAGGAGAGGGCTGAGGT
14.3 If the sequence of base pairs along a DNA molecule
occurs strictly at random, what is the expected frequency
of a specic restriction enzyme recognition sequence of
length (a) four and (b) six base pairs?
ANS: (a) (1/4)
4
1/256; (b) (1/4)
6
1/4096
14.4 In what ways do restriction endonucleases differ from
other endonucleases?
ANS: Restriction endonucleases recognize and cut specic
nucleotide sequences in DNA. Most other endonucleases
are not sequence-specic; many cut DNA sequences at
random.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 56 8/14/2015 6:42:58 PM

Answers to All Questions and Problems WC-57
14.5 Of what value are recombinant DNA and gene-cloning
technologies to geneticists?
ANS: Recombinant DNA and gene-cloning techniques allow
geneticists to isolate essentially any gene or DNA
sequence of interest and to characterize it structurally
and functionally. Large quantities of a given gene can be
obtained in pure form, which permits one to determine
its nucleotide-pair sequence (to “sequence it” in com-
mon lab jargon). From the nucleotide sequence and our
knowledge of the genetic code, geneticists can predict
the amino acid sequence of any polypeptide encoded by
the gene. By using an appropriate subclone of the gene as
a hybridization probe in northern blot analyses, geneti-
cists can identify the tissues in which the gene is
expressed. Based on the predicted amino acid sequence
of a polypeptide encoded by a gene, geneticists can syn-
thesize oligopeptides and use these to raise antibodies
that, in turn, can be used to identify the actual product of
the gene and localize it within cells or tissues of the
organism. Thus, recombinant DNA and gene-cloning
technologies provide very powerful tools with which to
study the genetic control of essentially all biological pro-
cesses. These tools have played major roles in the explo-
sive progress in the eld of biology during the last three
decades.
14.6 What determines the sites at which DNA molecules will
be cleaved by a restriction endonuclease?
ANS: The nucleotide-pair sequence. Restriction endonucle-
ases recognize a specic nucleotide-pair sequence in
DNA regardless of the source of the DNA. In most cases,
this is a 4 or 6 nucleotide-pair sequence; in a few cases,
the recognition sequence is longer (e.g., 8 nucleotide
pairs). Most restriction enzymes cleave the two strands
of the DNA at a specic position (between the same two
adjacent nucleotides in each strand) within the recogni-
tion sequence. A few restriction enzymes bind at a spe-
cic recognition sequence but cut the DNA at a nearby
site outside of the recognition sequences. Some restric-
tion endonucleases cut both strands between the same
two nucleotide pairs (“blunt end” cutters), whereas oth-
ers cut the two strands at different positions and yield
complementary single-stranded ends (“sticky or stag-
gered end” cutters). See Table 14.1 for examples.
14.7 Restriction endonucleases are invaluable tools for biolo-
gists. However, genes encoding restriction enzymes
obviously did not evolve to provide tools for scientists.
Of what possible value are restriction endonucleases to
the microorganisms that produce them?
ANS: Restriction endonucleases are believed to provide a kind
of primitive immune system to the microorganisms
that produce them—protecting their genetic material
from “invasion” by foreign DNAs from viruses or other
pathogens or just DNA in the environment that might
be taken up by the microorganism. Obviously, these
microorganisms do not have a sophisticated immune sys-
tem like that of higher animals (see Chapter 22).
14.8 Why is the DNA of a microorganism not degraded by a
restriction endonuclease that it produces, even though
its DNA contains recognition sequences normally
cleaved by the endonuclease?
ANS: Microorganisms that produce restriction endonucleases
also produce enzymes that modify one or more bases in
the recognition sequence for that endonuclease so that it
can no longer cleave the DNA at that site. In most cases,
the modifying enzyme is a methylase that attaches a
methyl group to one or more of the bases in the recogni-
tion sequence. For example, E. coli strains that produce
the restriction endonuclease EcoRI also produce EcoRI
methylase, an enzyme that transfers a methyl group from
S-adenosylmethionine to the second (most 3′) adenine
residue in each strand of the recognition sequence
(5-GAATCC-3) producing N
6
-methyladenines at these
positions. EcoRI cannot cleave DNA that contains
N
6
-methyladenine at these positions even if the EcoRI
recognition sequence is present in this DNA (see Figure
14.1). Thus, if one wishes to digest DNA with EcoRI, that
DNA must not be isolated from an E. coli strain that is
producing EcoRI methylase.
14.9 One of the procedures for cloning foreign DNA segments
takes advantage of restriction endonucleases such as Hin-
dIII (see Table 14.1) that produce complementary single-
stranded ends. These enzymes produce identical
complementary ends on cleaved foreign DNAs and on the
vector DNAs into which the foreign DNAs are inserted.
Assume that you have inserted your favorite gene into the
HindIII site in the polycloning region of the Bluescript
cloning vector with DNA ligase, have amplied the plas-
mid containing your gene in E. coli, and have isolated a
large quantity of gene/Bluescript DNA. How could you
excise your favorite gene from the Bluescript vector?
ANS: A foreign DNA cloned using an enzyme that produces
single-stranded complementary ends can always be
excised from the cloning vector by cleavage with the
same restriction enzyme that was originally used to clone
it. For example, the HindIII fragment carrying your
favorite can be excised from the Bluescript DNA by
cleavage with restriction endonuclease HindIII. The
human HindIII fragment will be anked in the recombi-
nant plasmid DNA clone by HindIII cleavage sites.
14.10 You are working as part of a research team studying the
structure and function of a particular gene. Your job is to
clone the gene. A restriction map is available for the
region of the chromosome in which the gene is located;
the map is as follows:
Xba
I PstI
HindIII
EcoRIEcoRI Hind
III
SalI
GENE
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 57 8/14/2015 6:42:58 PM
58-WC Answers to All Questions and Problems
Your rst task is to prepare a genomic DNA library that
contains clones carrying the entire gene. Describe how
you would prepare such a library in plasmid vector
pBluescript II (see Figure 14.3), indicating which restric-
tion enzymes, media, and host cells you would use.
ANS: Step 1: Digest genomic DNA isolated from your research
organism with EcoRI. Step 2: Treat pBluescript II DNA
with EcoRI. Step 3: Mix the EcoRI-digested genomic and
vector DNAs under annealing conditions and incubate
with DNA ligase. Step 4: Transform amp
5
E. coli cells car-
rying the lacZM15 gene with the resulting ligation prod-
ucts. Step 5: Plate the transformed cells on nutrient agar
medium containing Xgal and ampicillin. Only trans-
formed cells will produce colonies in the presence of
ampicillin. Step 6: Prepare your genomic DNA library
by using bacteria from white colonies; these bacteria will
contain pBluescript II DNA with genomic DNA inserts.
Bacteria harboring Bluescript II plasmids with no insert
will produce blue colonies (see Figure 14.4).
14.11 Compare the nucleotide-pair sequences of genomic
DNA clones and cDNA clones of specic genes of higher
plants and animals. What is the most frequent difference
that you would observe?
ANS: Most genes of higher plants and animals contain non-
coding intron sequences. These intron sequences will be
present in genomic clones, but not in cDNA clones,
because cDNAs are synthesized using mRNA templates
and intron sequences are removed during the processing
of the primary transcripts to produce mature mRNAs.
14.12 Most of the genes of plants and animals that were cloned
soon after the development of recombinant DNA tech-
nologies were genes encoding products that are synthe-
sized in large quantities in specialized cells. For example,
about 90 percent of the protein synthesized in mature
red blood cells of mammals consists of - and b-globin
chains, and the globin genes were among the rst mam-
malian genes cloned. Why were genes of this type so
prevalent among the rst eukaryotic genes that were
cloned?
ANS: Higher eukaryotes have very large genomes; for example,
the genomes of mammals contain approximately 3 10
9
nucleotide pairs. Thus, trying to identify a particular
single-copy gene from a clone library is like looking for
the proverbial “needle-in-a-haystack.” To accomplish
this, one needs a nucleic acid hybridization probe specic
for the gene or an antibody probe specic for the gene
product. Given a specic cell or tissue type producing the
mRNA and/or the protein gene product in large amounts,
it was relatively easy to obtain pure mRNA or pure pro-
tein to use in making a hybridization or antibody probe,
respectively, with which to screen a library for the gene or
cDNA of interest. These approaches are much more dif-
cult for the majority of the genes that encode products
that represent only a small proportion of the total gene
products in any given cell type.
14.13 Genomic clones of the chloroplastic glutamine synthe-
tase gene (gln2) of maize are cleaved into two fragments
by digestion with restriction endonuclease HindIII,
whereas full-length maize gln2 cDNA clones are not cut
by HindIII. Explain these results.
ANS: The maize gln2 gene contains many introns, and one of
the introns contains a HindIII cleavage site. The intron
sequences (and thus the HindIII cleavage site) are not
present in mRNA sequences and thus are also not pres-
ent in full-length gln2 cDNA clones.
14.14 In the following illustration, the upper line shows a gene
composed of segments A–D. The lower circle shows a
mutant version of this gene, consisting of two fused
pieces (A-B, C-D), carried on a plasmid. You attempt a
targeted mutagenesis of a diploid cell by transforming
cells with the cloned mutant gene. The following dia-
gram shows the desired pairing of the plasmid and chro-
mosome just prior to recombination.
ABC
A' B' C'
Probe
Plasmid
D
D'
x
xx
x
You prepare DNA from the cells, digest it with an enzyme
that cuts at x, and hybridize the cleaved DNA with the
probe shown above. The following diagram shows a
Southern blot of possible results.
1 2 3 4 5
____ ____ ____ ____
____ ____
____
(a) Which lane shows fragments produced from DNA in
the cell before transformation? (b) Which lane shows
fragments produced from DNA in the cell in which the
anticipated targeted mutagenesis occurred?
(c) Which of these blot patterns might be expected if two
crossovers occurred, one between A and B, and the other
between C and D?
ANS: (a) 1 (b) 2 (c) 2
14.15 (a) What experimental procedure is carried out in South-
ern, northern, and western blot analyses? (b) What is the
major difference between Southern, northern, and west-
ern blot analyses?
ANS: (a) Southern, northern, and western blot procedures
all share one common step, namely, the transfer of
macromolecules (DNAs, RNAs, and proteins, respec-
tively) that have been separated by gel electrophoresis
to a solid support—usually a nitrocellulose or nylon
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 58 8/14/2015 6:42:59 PM
Answers to All Questions and Problems WC-59
membrane—for further analysis. (b) The major difference
between these techniques is the class of macromolecules
that are separated during the electrophoresis step: DNA
for Southern blots, RNA for northern blots, and protein
for western blots.
14.16 What major advantage does the polymerase chain reac-
tion (PCR) have over other methods for analyzing
nucleic acid structure and function?
ANS: The PCR technique has much greater sensitivity than
any other method available for analyzing nucleic acids.
Thus, PCR procedures permit analysis of nucleic acid
structure given extremely minute amounts of starting
material. DNA sequences can be amplied and structur-
ally analyzed from very small amounts of tissue like
blood or sperm in assault and rape cases. In addition,
PCR methods permit investigators to detect the pres-
ence of rare gene transcripts (e.g., in specic types of
cells) that could not be detected by less sensitive proce-
dures such as northern blot analyses or in situ hybridiza-
tion studies.
14.17 The cloning vectors in use today contain an origin of
replication, a selectable marker gene (usually an antibi-
otic-resistance gene), and one additional component.
What is this component, and what is its function?
ANS: All modern cloning vectors contain a “polycloning site”
or “multiple cloning site” (MCS)—a cluster of unique
cleavage sites for a number of different restriction endo-
nucleases in a nonessential region of the vector into
which the foreign DNA can be inserted. In general, the
greater the complexity of the MCS—that is, the more
restriction endonuclease cleavage sites that are present—
the greater the utility of the vector for cloning a wide
variety of different restriction fragments. For example,
see the MCS present in plasmid Bluescript II shown in
Figure 14.3.
14.18 The drawing in this problem shows a restriction map of
a segment of a DNA molecule. Eco refers to locations
where the restriction endonuclease EcoRI cuts the DNA,
and Pst refers to locations where the restriction enzyme
PstI cuts the DNA. Potential restriction sites are num-
bered 1–6. Distances between restriction sites are shown
on the bottom scale in base pairs (bp). The thick line
represents the part of the molecule that has homology
with a probe.
Eco
5000 bp 3000 bp 4000 bp 5000 bp2000 bp
1
Pst
2
Eco
3
Pst
4
Eco
5
Pst
6
(a) Assume that individual 1 has restriction sites 1 through
6. If this individual’s DNA is digested with PstI, what are
the expected sizes of the DNA fragments that will
hybridize with the probe? (b) Assume that individual 2
has a mutation that eliminates site 4. If this individual’s
DNA is digested with PstI, what are the expected sizes of
the DNA fragments that will hybridize with the probe?
(c) Assume that individual 3 has a mutation that elimi-
nates site 5. If the DNA of this individual is digested with
PstI, what are the expected sizes of the DNA fragments
that will hybridize with the probe? (d) If the DNA of
individual 1 is digested with both PstI and EcoRI, what
are the expected sizes of the DNA fragments that will
hybridize with the probe? (e) If the DNA of individual 3
is digested with both PstI and EcoRI, what are the
expected sizes of the DNA fragments that will hybridize
with the probe?
ANS: (a) 7000 bp 7000 bp; (b) 14,000 bp; (c) 7000 bp 7000
bp; (d) 4000 bp 2000 bp 5000 bp; (e) 4000 bp
7000 bp.
14.19 The cystic brosis (CF) gene (location: chromosome 7,
region q31) has been cloned and sequenced, and studies
of CF patients have shown that about 70 percent of them
are homozygous for a mutant CF allele that has a specic
three-nucleotide-pair deletion (equivalent to one codon).
This deletion results in the loss of a phenylalanine residue
at position 508 in the predicted CF gene product. Assume
that you are a genetic counselor responsible for advising
families with CF in their pedigrees regarding the risk of
CF among their offspring. How might you screen puta-
tive CF patients and their parents and relatives for the
presence of the CFDF508 mutant gene? What would the
detection of this mutant gene in a family allow you to say
about the chances that CF will occur again in the family?
ANS: Because the nucleotide-pair sequences of both the normal
CF gene and the CF∆508 mutant gene are known, labeled
oligonucleotides can be synthesized and used as hybridiza-
tion probes to detect the presence of each allele (normal
and ∆508). Under high-stringency hybridization condi-
tions, each probe will hybridize only with the CF allele that
exhibits perfect complementarity to itself. Since the
sequences of the CF gene anking the ∆508 site are known,
oligonucleotide PCR primers can be synthesized and used
to amplify this segment of the DNA obtained from small
tissue explants of putative CF patients and their relatives
by PCR. The amplied DNAs can then be separated
by agarose gel electrophoresis, transferred to nylon
membranes, and hybridized to the respective labeled
oligonucleotide probes, and the presence of each CF allele
can be detected by autoradiography. For a demonstration
of the utility of this procedure, see Focus on Detection of
a Mutant Gene Causing Cystic Fibrosis. In the procedure
described there, two synthetic oligonucleotide probes—
oligo-N 3′-CTTTTATAGTAGAAACCAC-5′ and
oligo-DF 3′-TTCTTTTATAGTA—ACCACAA-5′
(the dash indicates the deleted nucleotides in the CFD508
mutant allele) were used to analyze the DNA of CF
patients and their parents. For conrmed CF families, the
results of these Southern blot hybridizations with the
oligo-N (normal) and oligo-DF (CFD508) labeled probes
were often as follows:
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 59 8/14/2015 6:42:59 PM

60-WC Answers to All Questions and Problems
Oligo-
∆F probe:
Oligo-N probe:
Both parents were heterozygous for the normal CF allele
and the mutant CFD508 allele as would be expected for a
rare recessive trait, and the CF patient was homozygous for
the CFD508 allele. In such families, one-fourth of the chil-
dren would be expected to be homozygous for the D508
mutant allele and exhibit the symptoms of CF, whereas
three-fourths would be normal (not have CF). However,
two-thirds of these normal children would be expected to
be heterozygous and transmit the allele to their children.
Only one-fourth of the children of this family would be
homozygous for the normal CF allele and have no chance
of transmitting the mutant CF gene to their offspring.
Note that the screening procedure described here can be
used to determine which of the normal children are carri-
ers of the CFD508 allele: that is, the mutant gene can be
detected in heterozygotes as well as in homozygotes.
14.20 Cereal grains are major food sources for humans and
other animals in many regions of the world. However,
most cereal grains contain inadequate supplies of certain
of the amino acids that are essential for monogastric ani-
mals such as humans. For example, corn contains insuf-
cient amounts of lysine, tryptophan, and threonine.
Thus, a major goal of plant geneticists is to produce corn
varieties with increased kernel lysine content. As a pre-
requisite to the engineering of high-lysine corn, molecu-
lar biologists need more basic information about the
regulation of the biosynthesis and the activity of the
enzymes involved in the synthesis of lysine. The rst step
in the anabolic pathway unique to the biosynthesis of
lysine is catalyzed by the enzyme dihydrodipicolinate
synthase. Assume that you have recently been hired by a
major U.S. plant research institute and that you have
been asked to isolate a clone of the nucleic acid sequence
encoding dihydrodipicolinate synthase in maize. Briey
describe four different approaches you might take in
attempting to isolate such a clone and include at least
one genetic approach.
ANS: You could attempt to isolate either a dihydropicolinate
synthase (DHPS) cDNA clone or a DHPS genomic
clone. Once you have isolated either DHPS clone, it can
be used as a hybridization probe to isolate the other
(genomic or cDNA) by screening an appropriate library
by in situ colony hybridization. Four approaches that
have proven effective in isolating other eukaryotic cod-
ing sequences of interest are the following: (1) You could
obtain a clone of the DHPS gene of a lower eukaryote
(a clone of the DHPS gene of Saccharomyces cerevisae
is available) or even a prokaryote and use it as a heter-
ologous hybridization probe to screen a maize cDNA
library using low stringency conditions. Sometimes this
approach is successful; sometimes it is not successful.
Whether or not this approach works depends on how
similar the coding sequences of the specic gene of inter-
est are in the two species. (2) You could purify the DHPS
enzyme from corn and use the puried protein to pro-
duce an antibody to DHPS. This DHPS-specic anti-
body could then be used to screen a maize cDNA
expression library by a protocol analogous to the western
blot procedure. (An expression library contains the
cDNA coding sequences fused to appropriate transcrip-
tion and translation signals so that they are expressed in
E. coli or other host cells in which the cDNA library is
prepared.) (3) You could purify the DHPS enzyme from
maize and determine the amino acid sequence of its
NH
2
-terminus by microsequencing techniques. From
the amino acid sequence and the known genetic code,
you could predict the possible nucleotide sequences
encoding this segment of the protein. Because of the
degeneracy in the code, there would be a set of nucleo-
tide sequences that would all specify the same amino acid
sequence, and you would not know which one was pres-
ent in the maize DHPS gene. However, the synthesis of
oligonucleotides is now routine and quite inexpensive.
Thus, you could synthesize a mixture of oligonucleotides
containing all possible coding sequences and use this
mixture as a set of hybridization probes to screen an
appropriate library by in situ colony hybridization.
(4) Finally, you might try a simple and very quick genetic
approach based on the ability of cDNAs in an expression
library to rescue DHPS mutants of E. coli or other species
that can be transformed at high frequencies. You would
obtain a DHPS-decient mutant of E. coli (available from
the E. coli Genetics Stock Center at Yale University),
transform it with your cDNA expression library, and
plate the transformed cells on medium lacking diami-
nopimelic acid (the product of DHPS). DHPS-decient
E. coli mutants cannot grow in the absence of diaminopi-
melic acid; thus, any colonies that grow on your selection
plates should be the result of rescue of the DHPS mutant
bacteria by corn DHPS encoded by cDNAs in the library.
This entire screening procedure can be carried out in 3
or 4 days; thus, it is much simpler than the preceding
approaches. In fact, David A. Frisch rst isolated a maize
DHPS cDNA by this simple, but powerful genetic
approach. However, that this approach would only be
expected to work in the case of enzymes that are active as
monomers or homomultimers; it is not applicable when
the active form of the enzyme is a heteromultimer.
14.21 You have just isolated a mutant of the bacterium Shigella
dysenteriae that is resistant to the antibiotic kanamycin,
and you want to characterize the gene responsible for
this resistance. Design a protocol using genetic selection
to identify the gene of interest.
ANS: Genetic selection is the most efcient approach to clon-
ing genes of this type. Prepare a genomic library in an
expression vector such as Bluescript (see Figure 14.3)
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 60 8/14/2015 6:42:59 PM
Answers to All Questions and Problems WC-61
using DNA from the kanamycin-resistant strain of Shi-
gella dysenteriae. Then, screen the library for the kanamy-
cin-resistance gene by transforming kanamycin-sensitive
E. coli cells with the clones in the library and plating the
transformed cells on medium containing kanamycin.
Only cells that are transformed with the kanamycin-
resistance gene will produce colonies in the presence of
kanamycin.
14.22 You have isolated a cDNA clone encoding a protein of
interest in a higher eukaryote. This cDNA clone is not
cleaved by restriction endonuclease EcoRI. When this
cDNA is used as a radioactive probe for blot hybridiza-
tion analysis of EcoRI-digested genomic DNA, three
radioactive bands are seen on the resulting Southern
blot. Does this result indicate that the genome of the
eukaryote in question contains three copies of the gene
encoding the protein of interest?
ANS: No. The genome of the species in question may contain
one, two, or three copies of the gene (or family of closely
related genes) encoding this protein. The possibilities
are as follows:
(1) One copy of the gene with two EcoRI cleavage sites
located within intron sequences. (2) Two copies of the
gene with one EcoRI cleavage site located within an
intron sequence of one of the copies. (3) Three copies of
the gene with no EcoRI cleavage site in any of the copies,
that is, each copy present on a single EcoRI restriction
fragment.
14.23 A linear DNA molecule is subjected to single and double
digestions with restriction endonucleases, and the fol-
lowing results are obtained:
Enzymes Fragment Sizes (in kb)
EcoRI 2.9, 4.5, 7.4, 8.0
HindIII 3.9, 6.0, 12.9
EcoRI and HindIII 1.0, 2.0, 2.9, 3.5, 6.0, 7.4
Draw the restriction map dened by these data.
ANS:
HH
E
6.0 2.0 7.4 3.5
1.0
2.9
EE
14.24 A DNA molecule is subjected to single and double diges-
tions with restriction enzymes, and the products are sep-
arated by gel electrophoresis. The results are as follows
(fragment sizes are in kb):
EcoRI
EcoRI
and
HindIII HindIII BamHI
EcoRI
and
BamHI
HindIII
and
BamHI
8 5 12 6 6 6
4 4 6 4 5
3 2 1
Draw the restriction map of this DNA molecule.
ANS:
H
B
B
1
2
4
5
E
E
14.25 You are studying a circular plasmid DNA molecule of
size 10.5 kilobase pairs (kb). When you digest this plas-
mid with restriction endonucleases BamHI, EcoRI, and
HindIII, singly and in all possible combinations, you
obtain linear restriction fragments of the following sizes:
Enzymes Fragment Sizes (in kb)
BamHI 7.3, 3.2
EcoRI 10.5
HindIII 5.1, 3.4, 2.0
BamHI EcoRI
6.7, 3.2, 0.6
BamHI HindIII
4.6, 2.7, 2.0, 0.7, 0.5
EcoRI HindIII
4.0, 3.4, 2.0, 1.1
BamHI EcoRI HindIII
4.0, 2.7, 2.0, 0.7, 0.6, 0.5
Draw a restriction map for the plasmid that ts your
data.
ANS: There are two possible restriction maps for these data as
shown below:
E
B
H
B
H
H
4.0
2.0
0.7
2.7
0.5
0.6
E
B
H
B
H
H
4.0
2.7
0.7
2.0
0.5
0.6
Restriction enzyme cleavage sites for BamHI, EcoRI, and
HindIII are denoted by B, E, and H, respectively. The
numbers give distances in kilobase pairs.
14.26 The automated DNA sequencing machines utilize uo-
rescent dyes to detect the nascent DNA chains synthe-
sized in the presence of the four dideoxy (ddX) chain
terminators, each labeled with a different uorescent
dye. The dyes uoresce at different wavelengths, which
are recorded by a photocell as the products of the reac-
tions are separated based on length by capillary gel elec-
trophoresis (see Figure 14.17). In the standard sequencing
reaction, the chains terminating with ddG uoresce dark
blue (peaks appear black in computer printout), those
terminating with ddC uoresce light blue, those termi-
nating with ddA uoresce green, and those terminating
with ddT uoresce red. The computer printout for the
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 61 8/14/2015 6:43:00 PM
62-WC Answers to All Questions and Problems
sequence of a short segment of DNA is shown as
follows.
First
nucleotide
Last
nucleotide
What is the nucleotide sequence of the nascent strand of
DNA?
What is the nucleotide sequence of the DNA template
strand?
ANS: Nascent strand:
5-AGTTCTAGAGCGGCCGCCACCGCGTGGAGCTCCAGCTTTTGTTCCCTTT-3
Template strand:
3-TCAAGATCTCGCCGGCGGTGGCGCACCTCGAGGTCGAAAACAAGGGAAA-5
14.27 Ten micrograms of a decanucleotide-pair HpaI restriction
fragment were isolated from the double-stranded DNA
chromosome of a small virus. Octanucleotide poly(A)
tails were then added to the 3′ ends of both strands using
terminal transferase and dATP; that is,
5′-X X X X X X X X X X-3′
3′-X′ X′ X′ X′ X′ X′ X′ X′ X′ X′-5′
↓ terminal transferase, dATP
5′-X X X X X X X X X X A A A A A A A A-3′
3′-A A A A A A A A X′ X′ X′ X′ X′ X′ X′ X′ X′ X′-5′
where X and X′ can be any of the four standard nucleo-
tides, but X′ is always complementary to X.
The two complementary strands (“Watson” strand and
“Crick” strand) were then separated and sequenced by
the 2′,3′-dideoxyribonucleoside triphosphate chain-ter-
mination method. The reactions were primed using a
synthetic poly(T) octamer; that is,
Watson strand
3′-A A A A A A A A X′ X′ X′ X′ X′ X′ X′ X′ X′ X′-5′
5′-T T T T T T T T-OH
Crick strand
5′-X X X X X X X X X X A A A A A A A A-3′
HO-T T T T T T T T-5′
Two DNA sequencing reactions were carried out.
Reaction 1 contained the Watson strand template/primer
shown above; reaction 2 contained the Crick strand
template/primer. Both sequencing reactions contained
DNA polymerase and all other substrates and compo-
nents required for DNA synthesis in vitro plus the
standard four 2′,3′-dideoxyribonucleoside triphosphate
chain-terminators—ddGTP, ddCTP, ddATP, and ddTTP—
each labeled with a different uorescent dye. The dyes
uoresce at different wavelengths, which are recorded by
a photocell as the products of the reactions are separated
by capillary gel electrophoresis (see Figure 14.17). In the
standard sequencing reaction, the chains terminating with
ddG uoresce dark blue (peaks appear black in the com-
puter printouts), those terminating with ddC uoresce
light blue, those terminating with ddA uoresce green,
and those terminating with ddT uoresce red. The com-
puter printout for sequencing reaction 1, which contained
the Watson strand as template, is shown as follows.
110Nucleotide:
Draw the predicted computer printout for reaction
2, which contained the Crick strand as template, in the
following box. Remember that all DNA synthesis occurs
in the 5′ → 3′ direction and that the sequence of
the nascent strand reads 5′ to 3′ from left to right in the
printout.
110Nucleotide:
ANS:
110Nucleotide:
Chapter 15
15.1 Distinguish between a genetic map, a cytogenetic map,
and a physical map. How can each of these types of maps
be used to identify a gene by positional cloning?
ANS: Genetic map distances are determined by crossover fre-
quencies. Cytogenetic maps are based on chromosome
morphology or physical features of chromosomes. Physi-
cal maps are based on actual physical distances—the
number of nucleotide pairs (0.34 nm per base pair)—
separating genetic markers. If a gene or other DNA
sequence of interest is shown to be located near a mutant
gene, a specic band on a chromosome, or a particular
DNA restriction fragment, that genetic or physical
marker (mutation, band, or restriction fragment) can be
used to initiate a chromosome walk to the gene of
interest.
15.2 In the technique of positional cloning, a researcher
begins with a DNA library and selects a clone that is
tightly linked to the gene of interest. That clone, or a
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 62 8/14/2015 6:43:00 PM

Answers to All Questions and Problems WC-63
piece of it, is then used as a probe to isolate an overlap-
ping clone from a different DNA library. The second
clone is used to isolate a third overlapping clone from
the rst library, and so on, until the researcher has
“walked” along the chromosome to the desired locus. (a)
How can the researcher walk consistently in the same
direction along the chromosome during the cloning pro-
cess? (b) What could happen if a long repetitive DNA
sequence such as a transposon was situated between the
starting clone and the gene of interest?
ANS: (a) To ensure that the researcher “walks” in the same
direction along the chromosome to the desired locus, it is
necessary to check at each step in the process, usually by
blot hybridization with subclones, which end of the clone
used as a probe overlaps with the next recovered clone.
(b) A long repetitive sequence could present a problem in
chromosome walking because if that sequence is used as a
probe to isolate a new clone, it will hybridize with restric-
tion fragments from all over the genome, and thereby
thwart the effort to walk systematically in one direction
along a single chromosome. To overcome this problem, a
researcher would have to “jump over” the repetitive ele-
ment and use a piece of unique DNA beyond the element
as a probe in the next step of the walking procedure.
15.3 What is a contig? What is an RFLP? What is a VNTR?
What is an STS? What is an EST? How is each of these
used in the construction of chromosome maps?
ANS: A contig (contiguous clones) is a physical map of a chro-
mosome or part of a chromosome prepared from a set of
overlapping genomic DNA clones. An RFLP (restriction
fragment length polymorphism) is a variation in the
length of a specic restriction fragment excised from a
chromosome by digestion with one or more restriction
endonucleases. A VNTR (variable number tandem
repeat) is a short DNA sequence that is present in the
genome as tandem repeats and in highly variable copy
number. An STS (sequence tagged site) is a unique DNA
sequence that has been mapped to a specic site on a
chromosome. An EST (expressed sequence tag) is a cDNA
sequence—a genomic sequence that is transcribed. Con-
tig maps permit researchers to obtain clones harboring
genes of interest directly from DNA Stock Centers—to
“clone by phone.” RFLPs are used to construct the high-
density genetic maps that are needed for positional clon-
ing. VNTRs are especially valuable RFLPs that are used
to identify multiple sites in genomes. STSs and ESTs
provide molecular probes that can be used to initiate
chromosome walks to nearby genes of interest.
15.4 The following is a Southern blot of EcoRI-digested DNA
of rye plants from two different inbred lines, A and B.
Developed autoradiogram I shows the bands resulting
from probing the blot with
32
P-labeled cDNA1. Autora-
diogram II shows the same Southern blot after it was
stripped of probe and reprobed with
32
P-labeled cDNA2.
I II
A B A B
a1
b1
b2
a2
a3 b3
a4
(a) Which bands would you expect to see in the autora-
diogram of a similarly probed Southern blot prepared
using EcoRI-digested DNA from F
1
hybrid plants pro-
duced by crossing the two inbred lines? (b) What can you
conclude about the gene(s) represented by band a1 on
blot I in the two inbreds? (c) The F
1
plants were crossed
to plants possessing only bands a1, a4, and b3. DNA was
isolated from several individual progeny and digested
with EcoRI. The resulting DNA fragments were sepa-
rated by gel electrophoresis, transferred to a nylon mem-
brane, and hybridized with radioactive cDNA1 and
cDNA2 probes. The following table summarizes the
bands present in autoradiograms obtained using DNA
from individual progeny.
Plant
No. Bands Present
a1 a2 a3 a4 b1 b2 b3
1
2
3
4
5
6
7
8
9
10
Interpret these data. Do the data provide evidence for
RFLPs? At how many loci? Are any of the RFLPs
linked? If so, what are the linkage distances dened by
the data?
ANS: (a) a1, a2, a3, a4, b1, b2, and b3. (b) Band a1 represents a
locus whose DNA is homologous to cDNA1. Since the
marker is not polymorphic in the parents used, it cannot
be mapped in this cross. (c) The cDNA1 probe detects
one RFLP locus with alleles that are visualized as band
a4 and bands a2/a3. The cDNA2 detects a second RFLP
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 63 8/14/2015 6:43:01 PM

64-WC Answers to All Questions and Problems
locus with alleles that are visualized as band b3 and bands
b1/b2. The two loci are linked with 20% recombination
observed in this cross.
15.5 As part of the Human Genome Mapping Project, you are
trying to clone a gene involved in colon cancer. Your rst
step is to localize the gene using RFLP markers. In the
following table, RFLP loci are dened by STS number
(e.g., STS1), and the gene for colon cancer is designated C.
Loci
% Recombi-
nation Loci
% Recombi-
nation
C, STS1 50 STS1, STS5 10
C, STS2 15 STS2, STS3 30
C, STS3 15 STS2, STS4 14
C, STS4 1 STS2, STS5 50
C, STS5 40 STS3, STS4 16
STS1, STS2 50 STS3, STS5 25
STS1, STS3 35 STS4, STS5 41
STS1, STS4 50
(a) Given the percentage recombination between differ-
ent RFLP loci and the gene for colon cancer shown in the
table, draw a genetic map showing the order and genetic
distances between adjacent RFLP markers and the gene
for colon cancer. (b) Given that the human genome con-
tains approximately 3.3 10
9
base pairs of DNA and that
the human genetic map contains approximately 3300 cM,
approximately how many base pairs of DNA are located
along the stretch of chromosome dened by this RFLP
map? (Hint: First gure how many base pairs of DNA are
present per cM in the human genome.) (c) How many
base pairs of DNA are present in the region between the
colon cancer gene and the nearest STS?
ANS: (a)
10 cM 25 cM 15 cM 1 cM 14 cM
STS1 STS5 STS3 C STS4 STS2
(b) 3.3 10
9
bp/3.3 10
3
cM 1 10
6
bp/cM. The
total map length is 65 cM, which equates to about 65
10
6
or 65 million bp.
(c) The cancer gene (C) and STS4 are separated by 1 cM
or about 1 million base pairs.
15.6 What are STRs? Why are they sometimes called
microsatellites?
ANS: STRs are polymorphic tandem repeats of sequences of
only two to four nucleotide pairs. They are called micro-
satellites because they are short in length and are compo-
nents of the highly repetitive satellite DNAs of eukaryotes
(see Chapter 9).
15.7 You have cloned a previously unknown human gene.
What procedure will allow you to position this gene on
the cytological map of the human genome without per-
forming any pedigree analyses? Describe how you would
carry out this procedure.
ANS: With a clone of the gene available, uorescent in situ
hybridization (FISH) can be used to determine which
human chromosome carries the gene and to localize the
gene on the chromosome. Single-stranded copies of the
clone are coupled to a uorescent probe and hybridized
to denatured DNA in chromosomes spread on a slide.
After hybridization, free probe is removed by washing,
and the location of the uorescent probe is determined
by photography using a uorescence microscope (see
Focus on: In Situ Hybridization).
15.8 You have identied a previously unknown human EST.
What must be done before this new EST can be called
an STS?
ANS: An EST must be placed on the physical map of a chro-
mosome before it can be called an STS. If it is also posi-
tioned on the genetic map of the chromosome, then it is
called an anchor marker.
15.9 VNTRs and STRs are specic classes of polymorphisms.
What is the difference between a VNTR and an STR?
ANS: Variable number tandem repeats (VNTRs) are com-
posed of repeated sequences of 10–80 nucleotide pairs,
and short tandem repeats (STRs) are composed of
repeated sequences of 2–10 nucleotide pairs.
15.10 An RFLP and a mutant allele that causes albinism in
humans cannot be shown to be separated by recombina-
tion based on pedigree analysis or by radiation hybrid
mapping. Do these observations mean that the RFLP
occurs within or overlaps the gene harboring the muta-
tion that causes albinism? If so, why? If not, why not?
ANS: The resolution of genetic mapping in humans is quite
low—in the range of 1–10 million base pairs. Radiation
hybrid mapping provides higher resolution—to about
50 kb. However, even with 50-kb resolution, there could
be several genes separating the RFLP from the mutation
responsible for albinism.
15.11 A cloned 6-kb fragment of DNA from human chromo-
some 9 contains a single site recognized by the restric-
tion enzyme EcoRI. This cloned fragment is demarcated
by sites for the restriction enzyme BamHI. There are no
other BamHI recognition sites within the clone.
A researcher has collected DNA samples from 10 people.
He digests each sample with a combination of EcoRI and
BamHI enzymes. The doubly digested DNA is then frac-
tionated by gel electrophoresis and blotted to a mem-
brane. After xing the DNA to the membrane, the
researcher hybridizes it with a radioactive probe made
from the entire cloned BamHI fragment. The autoradio-
gram obtained by exposing an X-ray lm to this mem-
brane yielded the following results. Three of the DNA
samples contained a 4-kb fragment and a 2-kb fragment
that hybridize with the probe, three of the DNA samples
contained a 6-kb DNA fragment that hybridizes with the
probe, and four of the DNA samples contain 6-, 4-, and
2-kb DNA fragments that hybridize with the probe.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 64 8/14/2015 6:43:01 PM
Answers to All Questions and Problems WC-65
What has this analysis revealed? What are the genotypes
of the three different types of DNA samples?
ANS: The results of this analysis reveal that the EcoRI cleavage
site within the original 6-kb BamHI clone is polymorphic—
that is, it is present in some chromosomes 9 but absent in
others. We can represent these two types of chromo-
somes 9 as BEB (with the EcoRI site between anking
BamHI sites) and B-B (without the EcoRI site). The rst
three samples came from people who were homozygous
for the BEB version of chromosome 9, the next three
samples came from people who were homozygous for
the B-B version, and the last four samples came from
people who where heterozygous for the two versions—
that is, they had the genotype BEB/B-B. In the human
population that was sampled, the EcoRI site is therefore
the basis for a restriction fragment length polymorphism
(RFLP).
15.12 Both an RFLP and a mutation that causes deafness in
humans map to the same location on the same chromo-
some. How can you determine whether or not the
RFLP overlaps with the gene containing the deafness
mutation?
ANS: You can start a chromosome walk using the hybridization
probe that detects the RFLP. However, if a physical map
of this region of the chromosome already exists (see
Figure 15.5), a chromosome walk might not be neces-
sary. cDNAs can be used to locate candidate genes in the
region covered by the chromosome walk, and the
sequences of genes in individuals with this form of inher-
ited deafness can be compared with the sequences of
homologous genes of individuals with normal hearing,
looking for changes that would be expected to cause a
loss of gene function. The overall process is illustrated in
Figure 15.6.
15.13 What were the goals of the Human Genome Project?
What impact has achieving these goals had on the prac-
tice of medicine to date? What are some of the predicted
future impacts? What are some of the possible misuses of
human genome data?
ANS: The goals of the Human Genome Project were to pre-
pare genetic and physical maps showing the locations of
all the genes in the human genome and to determine the
nucleotide sequences of all 24 chromosomes in the
human genome. These maps and nucleotide sequences
of the human chromosomes helped scientists identify
mutant genes that result in inherited diseases. Hopefully,
the identication of these mutant disease genes will lead
to successful treatments, including gene therapies, for at
least some of these diseases in the future. Potential mis-
uses of these data include invasions of privacy by govern-
ments and businesses—especially employment agencies
and insurance companies. Individuals must not be denied
educational opportunities, employment, or insurance
because of inherited diseases or mutant genes that result
in a predisposition to mental or physical abnormalities.
15.14 What difculty does repetitive DNA pose for the assem-
bly of whole genome shotgun sequences by computer
analysis?
ANS: Repetitive DNA can make it difcult to assemble long
stretches of DNA sequence from the sequences of DNA
fragments that contain these sequences. The reason is
that in the computer analysis, a repetitive sequence in
one DNA fragment will match with the same repetitive
sequence in another DNA fragment even if the two
DNA fragments are not contiguous in the genome. The
effort to determine which short DNA fragments are
authentic neighbors (contiguous to each other) can
therefore be thrown off by these spurious matches.
15.15 Which type of molecular marker, RFLP or EST, is most
likely to mark a disease-causing mutant gene in humans?
Why?
ANS: An EST is more likely than an RFLP to occur in a dis-
ease-causing human gene. All ESTs correspond to
expressed sequences in a genome. RFLPs occur through-
out a genome, in both expressed and unexpressed
sequences. Because less than 2 percent of the human
genome encodes proteins, most RFLPs occur in noncod-
ing DNA.
15.16 Bacteriophage ΦX174 contains 11 genes in a genome of
5386 bp; E. coli has a predicted 4288 genes in a genome
of about 4.639 kb; S. cerevisiae has about 6000 genes in a
genome of size 12.1 mb; C. elegans has about 19,000
genes present in a genome of about 100 mb; and H. sapi-
ens has an estimated 22,000 genes in its 3000-mb genome.
Which genome has the highest gene density? Which
genome has the lowest gene density? Does there appear
to be any correlation between gene density and develop-
mental complexity? If so, describe the correlation.
ANS: The bacteriophage ΦX174 genome has the highest gene
density—one gene per 490 nucleotide pairs. The human
genome has the lowest gene density—about one gene
per 100 kb. In the species mentioned in this question,
there is a striking correlation between genome size and
developmental complexity. With some exceptions,
including species with polyploid genomes, there does
appear to be a rough correlation between genome size
and developmental complexity.
15.17 A contig map of one segment of chromosome 3 of Arabi-
dopsis is as follows.
A
12345
Chromosome segment
678910
BH
CG
E
DF
Genomic clones in
YAC vectors
(a) If an EST hybridizes with genomic clones C, D, and
E, but not with the other clones, in which segment of
chromosome 3 is the EST located? (b) If a clone of gene
ARA hybridizes only with genomic clones C and D, in
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 65 8/14/2015 6:43:01 PM
66-WC Answers to All Questions and Problems
which chromosome segment is the gene located? (c) If a
restriction fragment hybridizes with only one of the
genomic clones shown above, in which chromosome
segment(s) could the fragment be located?
ANS: (a) Segment 5; (b) segment 4; (c) segment 1, 6, or 10.
15.18 Eight human–Chinese hamster radiation hybrids were
tested for the presence of six human ESTs designated A
through F. The results are shown in the following table,
where a plus indicates that a marker was present and a
minus indicates that it was absent.
12345678
––
––
––
––
– –––
––
––
––
––
–
A
B
C
D
E
F–
++
++
++
++
+++ +
++
++
++
++
+ ++++–
Radiation hybrid
Marker
Based on these data, do any of the ESTs appear to be
closely linked? Which ones? What would be needed for
you to be more certain of your answer?
ANS: EST markers D and E appear to be closely linked. The
eight human–Chinese hamster radiation hybrids contain
either both D and E or neither marker. More radiation
hybrids would need to be tested for the presence of these
ESTs to obtain convincing evidence of this linkage.
15.19 What is the major advantage of gene chips as a microar-
ray hybridization tool?
ANS: The major advantage of gene chips as a microarray
hybridization tool is that a single gene chip can be used
to quantify thousands of distinct nucleotide sequences
simultaneously. The gene-chip technology allows
researchers to investigate the levels of expression of a
large number of genes more efciently than was possible
using earlier microarray procedures.
15.20 What major advantage does the green uorescent pro-
tein of the jellysh have over other methods for studying
protein synthesis and localization?
ANS: The green uorescent protein (GFP) can be used to study
protein localization and movement over time in living
cells. Most other procedures for studying protein localiza-
tion require that cells be permeabilized and/or xed and
exposed to antibodies coupled to radioactive or uores-
cent compounds prior to visualization. As a result, these
procedures only provide information about the location
of a protein at a single time point. In contrast, GFP-tagged
proteins can be used to study the synthesis and movement
of proteins in living cells over time (hours to days).
15.21 You are given chromosome-specic cDNA libraries for
all 24 human chromosomes. How might these libraries
be used to study chromosome evolution in primates?
ANS: The DNA sequences in human chromosome-specic
cDNA libraries can be coupled to uorescent dyes and
hybridized in situ to the chromosomes of other primates.
The hybridization patterns can be used to detect changes
in genome structure that have occurred during the evo-
lution of the various species of primates from common
ancestors (see Figure 6.4). Such comparisons are espe-
cially effective in detecting new linkage relationships
resulting from translocations and centric fusions.
15.22 Of the cereal grass species, only maize contains two cop-
ies of each block of linked genes. What does this duplica-
tion of sets of maize genes indicate about the origin of
this agronomically important species?
ANS: The presence of two copies of each block of genes in the
corn genome indicates that maize has evolved from a tet-
raploid ancestor. The presence of one set of genes pri-
marily in the large chromosomes and the second set
largely in the small chromosomes suggests that maize
has evolved from an allotetraploid (see Chapter 6) pro-
duced by combining the diploid genomes of two ances-
tral cereal grass species.
15.23 Five human genomic DNA clones present in PAC vec-
tors were tested by hybridization for the presence of six
sequence-tagged sites designated STS1 through STS6.
The results are given in the following table: a plus indi-
cates the presence of the STS, and a minus indicates the
absence of the STS.
123456
+++
––+
––
+
++
–
––+
A
B
C
D
E
––
–
+
––
– ++
–––
– + –
STS
YAC clone
(a) What is the order of the STS sites on the
chromosome?
(b) Draw the contig map dened by these data.
ANS: (a) Order of STS sites: 2-5-1-4-3-6.
(b)
A
251436
B
C
DE
STS markers:
YAC
clones
15.24 The complete sequences of six mitochondrial genomes
of H. neanderthalensis have been available for some time;
the rst H. neanderthalensis mtDNA sequence was pub-
lished in 2008. How similar are the sequences of the
mtDNAs of H. neanderthalensis and H. sapiens? Are the
genomes similar in size? Is the amount of diversity
observed in the mtDNAs of Neanderthals and humans
the same? If not, what might this tell us about the sizes of
Neanderthal and human populations? How many genes
are present in the H. neanderthalensis mitochondrial
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 66 8/14/2015 6:43:02 PM

Answers to All Questions and Problems WC-67
genome? How many of these genes encode proteins?
How many specify structural RNA molecules? Are there
any pseudogenes in H. neanderthalensis mtDNA? All of
these questions can be answered by visiting the http://
www.ncbi.nlm.nih.gov/ web site.
ANS: The mtDNAs of Homo neanderthalensis and H. sapiens are
very similar, both in size and in nucleotide sequence. The
H. neanderthalensis mtDNA contains 16,565 nucleotide
pairs, whereas the H. sapiens mtDNA contains 16,569
nucleotide pairs, and the two DNAs exhibit 99 percent
sequence identity. There is less sequence diversity in the
mtDNAs of Neanderthals than in the mtDNAs of
humans, which is consistent with Neanderthal popula-
tions being smaller than human populations. There are
37 genes in the Neanderthal mtDNA: 13 encode pro-
teins and 24 specify structural RNAs (tRNAs and
rRNAs). The Neanderthal mitochondrial genome does
not contain any pseudogenes.
15.25 Assume that you have just sequenced a small fragment of
DNA that you had cloned. The nucleotide sequence of
this segment of DNA is as follows.
aagtagtcgaaaccgaattccgtagaaacaactcgcacgctccggtttc
gtgttgcaacaaaataggcattcccatcgcggcagttagaatcaccga
gtgcccagagtcacgttcgtaagcaggcgcagtttacaggcagca
gaaaaatcgattgaacagaaatggctggcggtaaagcaggcaagga
ttcgggcaaggccaaggcgaaggcggtatcgcgttccgcgcgcgcggg
In an attempt to learn something about the identity or
possible function of this DNA sequence, you decide to
perform a BLAST (nucleotide blast) search on the NCBI
web site (http://www.ncbi.nlm.nih.gov). Paste or type
this sequence into the query sequence box. Run the
search and examine the sequences most closely related to
your query sequence. Are they coding sequences? What
proteins do they encode? Repeat the BLAST search with
only half of your sequence as the query sequence. Do you
still identify the same sequences in the databases? If you
use one-fourth of your sequence as a query, do you still
retrieve the same sequences? What is the shortest DNA
sequence that you can use as a query and still identify the
same sequences in the databanks?
ANS: All of the sequences identied by the megablast search
encode histone H2a proteins. The query sequence is
identical to the coding sequence of the Drosophila
melanogaster histone H2aV gene (a member of the gene
family encoding histone H2a proteins). The query
sequence encodes a Drosophila histone H2a polypeptide
designated variant V. The same databank sequences are
identied when one-half or one-fourth of the given
nucleotide sequence is used as the query in the megablast
search. Query sequences as short as 15–20 nucleotides
can be used to identify the Drosophila gene encoding the
histone H2a variant. However, the results will vary
depending on the specic nucleotide sequence used as
the query sequence.
15.26 The NCBI web site (http://www.ncbi.nlm.nih.gov) can
also be used to search for protein sequences. Instead of
performing a BLAST search with a nucleic acid query,
one performs a protein blast with a polypeptide (amino
acid sequence) query. Assume that you have the follow-
ing partial sequence of a polypeptide:
GYDVEKNNSRIKLGLKSLVSKGILVQTKGT
GASGSFKLNKKAASGEAKPQAKKAGAAKA
Go to the NCBI web site and access the BLAST tool.
Then click on protein blast and enter the query sequence
in the box at the top. Then click BLAST. What is the
identity of your query sequence?
ANS: Your query sequence is a portion of histone H1.2 of the
mouse (Mus musculus). This portion of the mouse histone
H1.2 polypeptide differs by one amino acid from the
corresponding part of the human H1 histone.
15.27 The sequence of a gene in Drosophila melanogaster that
encodes a histone H2A polypeptide is as follows:
aagtagtcgaaaccgaattccgtagaaacaactcgcacgctccggtttc
gtgttgcaacaaaataggcattcccatcgcggcagttagaatcacc
gagtgcccagagtcacgttcgtaagcaggcgcagtttacaggcagcag
aaaaatcgattgaacagaaatggctggcggtaaagcaggcaagg
attcgggcaaggccaaggcgaaggcggtatcgcgttccgcgcg
cgcgggtcttcagttccccgtgggtcgcatccatcgtcatctcaag
agccgcactacgtcacatggacgcgtcggagccactgcagccgtg
tactccgctgccatattggaatacctgaccgccgaggtcctggagtt
ggcaggcaacgcatcgaaggacttgaaagtgaaacgtatcactcc
tcgccacttacagctcgccattcgcggagacgaggagctggacag
cctgatcaaggcaaccatcgctggtggcggtgtcattccgcacata
cacaagtcgctgatcggcaaaaaggaggaaacggtgcaggatccgc
agcggaagggcaacgtcattctgtcgcaggcctactaagccagtcgg
caatcggacgccttcgaaacatgcaacactaatgtttaattcagattt
cagcagagacaagctaaaacaccgacgagttgtaatcatttctgtgcg
ccagcatatatttcttatatacaacgtaatacataattatgtaattctagca
tctccccaacactcacatacatacaaacaaaaaatacaaacacacaaaac
gtatttacccgcacgcatccttggcgaggttgagtatgaaacaaa
aacaaaacttaatttagagcaaagtaattacacgaataaatttaataa
aaaaaactataataaaaacgcc.
Let’s use the translation software available on the Inter-
net at http://www.expasy.org/tools/dna.html to translate
this gene in all six possible reading frames and see which
reading frame species histone H2A. Just type or paste
the DNA sequence in the “ExPASy Translate” tool box
and click TRANSLATE SEQUENCE. The results will
show the products of translation in all six reading frames
with Met’s and Stop’s boldfaced to highlight potential
open-reading frames. Which reading frame species his-
tone H2A?
ANS: Reading frame 5′ → 3′ number 1 has a large open read-
ing frame with a methionine codon near the 5′ end. You
can verify that this is the correct reading frame by using
the predicted translation product as a query to search
one of the protein databases (see Problem 15.26).
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 67 8/14/2015 6:43:02 PM

68-WC Answers to All Questions and Problems
Chapter 16
16.1 What are CpG islands? Of what value are CpG islands in
positional cloning of human genes?
ANS: CpG islands are clusters of cytosines and guanines that
are often located just upstream (5′) from the coding
regions of human genes. Their presence in nucleotide
sequences can provide hints as to the location of genes in
human chromosomes.
16.2 Why is the mutant gene that causes Huntington’s disease
called huntingtin? Why might this gene be renamed in
the future?
ANS: The gene was named huntingtin after the disease that it
causes when defective. The gene will probably be
renamed after the function of its gene product has been
determined.
16.3 How was the nucleotide sequence of the CF gene used to
obtain information about the structure and function of
its gene product?
ANS: The CF gene was identied by map position-based clon-
ing, and the nucleotide sequences of CF cDNAs were
used to predict the amino acid sequence of the CF gene
product. A computer search of the protein data banks
revealed that the CF gene product was similar to several
ion channel proteins. This result focused the attention of
scientists studying cystic brosis on proteins involved in
the transport of salts between cells and led to the discov-
ery that the CF gene product was a transmembrane con-
ductance regulator—now called the CFTR protein.
16.4 How might the characterization of the CF gene and its
product lead to the treatment of cystic brosis by
somatic-cell gene therapy? What obstacles must be over-
come before cystic brosis can be treated successfully by
gene therapy?
ANS: Once the function of the CF gene product has been
established, scientists should be able to develop proce-
dures for introducing wild-type copies of the CF gene
into the appropriate cells of cystic brosis patients to
alleviate the devastating effects of the mutant gene. A
major obstacle to somatic-cell gene-therapy treatment of
cystic brosis is the size of the CF gene—about 250 kb,
which is too large to t in the standard gene transfer vec-
tors. Perhaps a shortened version of the gene constructed
from the CF cDNA—about 6.5 kb—can be used in place
of the wild-type gene. A second major obstacle is getting
the transgene into enough of the target cells of the cystic
brosis patient to alleviate the symptoms of the disease.
A third challenge is to develop an expression vector con-
taining the gene that will result in long-term expression
of the introduced gene in transgenic cells. Another con-
cern is how to avoid possible side effects caused by over-
expression or inappropriate expression of the transgene
in cystic brosis patients. Despite these obstacles, many
scientists are optimistic that cystic brosis will be effec-
tively treated by somatic-cell gene therapy in the future.
16.5 Myotonic dystrophy (MD), occurring in about 1 of 8000
individuals, is the most common form of muscular
dystrophy in adults. The disease, which is characterized
by progressive muscle degeneration, is caused by a domi-
nant mutant gene that contains an expanded CAG repeat
region. Wild-type alleles of the MD gene contain 5–30
copies of the trinucleotide. Mutant MD alleles contain
50 to over 2000 copies of the CAG repeat. The complete
nucleotide sequence of the MD gene is available. Design
a diagnostic test for the mutant gene responsible for
myotonic dystrophy that can be carried out using
genomic DNA from newborns, fetal cells obtained by
amniocentesis, and single cells from eight-cell pre-
embryos produced by in vitro fertilization.
ANS: Oligonucleotide primers complementary to DNA
sequences on both sides (upstream and downstream) of the
CAG repeat region in the MD gene can be synthesized and
used to amplify the repeat region by PCR. One primer
must be complementary to an upstream region of the tem-
plate strand, and the other primer must be complementary
to a downstream region of the nontemplate strand. After
amplication, the size(s) of the CAG repeat regions can be
determined by gel electrophoresis (see Figure 16.2). Tri-
nucleotide repeat lengths can be measured by including
repeat regions of known length on the gel. If fewer than 30
copies of the trinucleotide repeat are present on each chro-
mosome, the newborn, fetus, or pre-embryo is homozy-
gous for a wild-type MD allele or heterozygous for two
different wild-type MD alleles. If more than 50 copies of
the repeat are present on each of the homologous chromo-
somes, the individual, fetus, or cell is homozygous for a
dominant mutant MD allele or heterozygous for two dif-
ferent mutant alleles. If one chromosome contains less
than 30 copies of the CAG repeat and the homologous
chromosome contains more than 50 copies, the newborn,
fetus, or pre-embryo is heterozygous, carrying one
wild-type MD allele and one mutant MD allele.
16.6 In humans, the absence of an enzyme called purine
nucleoside phosphorylase (PNP) results in a severe
T-cell immunodeciency similar to that of severe com-
bined immunodeciency disease (SCID). PNP deciency
exhibits an autosomal recessive pattern of inheritance,
and the gene encoding human PNP has been cloned and
sequenced. Would PNP deciency be a good candidate
for treatment by gene therapy? Design a procedure for
the treatment of PNP deciency by somatic-cell gene
therapy.
ANS: Yes. A somatic-cell gene therapy procedure similar to
that used for X-linked SCID (see Figure 16.7) might be
effective in treating purine nucleoside phosphorylase
(PNP) deciency. White blood cells could be isolated
from the patient, transfected with a vector carrying a
wild-type PNP gene, grown in culture and assayed for
the expression of the PNP transgene, and then infused
back into the patient after the expression of the trans-
gene had been veried.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 68 8/14/2015 6:43:02 PM

Answers to All Questions and Problems WC-69
16.7 Human proteins can now be produced in bacteria such as
E. coli. However, one cannot simply introduce a human
gene into E. coli and expect it to be expressed. What steps
must be taken to construct an E. coli strain that will
produce a mammalian protein such as human growth
hormone?
ANS: The transcription initiation and termination and transla-
tion initiation signals or eukaryotes differ from those of
prokaryotes such as E. coli. Therefore, to produce a
human protein in E. coli, the coding sequence of the
human gene must be joined to appropriate E. coli regula-
tory signals—promoter, transcription terminator, and
translation initiator sequences. Moreover, if the gene
contains introns, they must be removed or the coding
sequence of a cDNA must be used, because E. coli does
not possess the spliceosomes required for the excision of
introns from nuclear gene transcripts. In addition, many
eukaryotic proteins undergo posttranslational process-
ing events that are not carried out in prokaryotic cells.
Such proteins are more easily produced in transgenic
eukaryotic cells growing in culture.
16.8 You have constructed a synthetic gene that encodes an
enzyme that degrades the herbicide glyphosate. You wish
to introduce your synthetic gene into Arabidopsis plants
and test the transgenic plants for resistance to glypho-
sate. How could you produce a transgenic Arabidopsis
plant harboring your synthetic gene by A. tumefaciens-
mediated transformation?
ANS: You would rst construct a chimeric gene containing
your synthetic gene fused to a plant promoter such as the
35S promoter of cauliower mosaic virus and a plant
transcription termination and polyadenylation signal
such as the one from the nos gene of the Ti plasmid. This
chimeric gene would then be inserted into the T-DNA
of a Ti plasmid carrying a dominant selectable marker
gene (e.g., 35S/NPTII/nos, which confers resistance to
kanamycin to host cells) and introduced into Agrobacte-
rium tumefaciens cells by transformation. Tissue explants
from Arabidopsis plants would be co-cultivated with A.
tumefaciens cells harboring the recombinant Ti plasmid,
and plant cells that carry T-DNAs inserted into their
chromosomes would be selected by growth on medium
containing the appropriate selective agent (e.g., kanamy-
cin). Transgenic plants would then be regenerated from
the transformed cells and tested for resistance to
glyphosate.
16.9 A human STR locus contains a tandem repeat (TAGA)
n
,
where n may be any number between 5 and 15. How
many alleles of this locus would you expect to nd in the
human population?
ANS: Eleven, ranging in multiples of 3, from 15 to 45 nucleo-
tides long.
16.10 A group of bodies are found buried in a forest. The police
suspect that they may include the missing Jones family
(two parents and two children). They extract DNA from
bones and examine (using PCR) genes A and B, which
are known to contain tandem triplet repeats of variable
length. They also analyze DNA from two other men.
The results are shown below where the numbers indi-
cate the number of copies of a tandem repeat in a par-
ticular allele; for example, male 1 has one allele with 8
and another allele with 9 copies of a tandem repeat in
gene A.
Gene A Gene B
Male 1
8/9 5/7
Male 2 6/8 5/5
Male 3 7/10 7/7
Woman 8/8 3/5
Child 1 7/8 5/7
Child 2 8/8 3/7
Could the woman have been the mother of both chil-
dren? Why or why not? Which man, if any, could have
been the father of child 1?
ANS: The woman could be the mother of both children, hav-
ing passed alleles A 8 and B 5 to child 1 and A 8 and B 3
to child 2. Male 3 could have been the father of child 1.
If so, he contributed alleles A 7 and B 7.
16.11 DNA proles have played central roles in many rape and
murder trials. What is a DNA prole? What roles do
DNA proles play in these forensic cases? In some cases,
geneticists have been concerned that DNA prole data
were being used improperly. What were some of their
concerns, and how can these concerns be properly
addressed?
ANS: DNA proles are the specic patterns (1) of peaks pres-
ent in electropherograms of chromosomal STRs or
VNTRs amplied by PCR using primers tagged with
uorescent dyes and separated by capillary gel electro-
phoresis (see Figures 16.11 and 16.12) or (2) of bands on
Southern blots of genomic DNAs that have been digested
with specic restriction enzymes and hybridized to
appropriate STR or VNTR sequences (see Figure 16.10).
DNA proles, “like” epidermal ngerprints, are used as
evidence for identity or nonidentity in forensic cases.
Geneticists have expressed concerns about the statistical
uses of DNA prole data. In particular, they have ques-
tioned some of the methods used to calculate the proba-
bility that DNA from someone other than the suspect
could have produced an observed prole. These con-
cerns have been based in part on the lack of adequate
DNA prole databases for various human subpopula-
tions and the lack of precise information about the
amount of variability in DNA proles for individuals of
different ethnic backgrounds. These concerns have been
addressed by the acquisition of data on prole frequen-
cies in different populations and ethnic groups from
throughout the world.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 69 8/14/2015 6:43:02 PM
70-WC Answers to All Questions and Problems
16.12 The DNA proles shown in this problem were prepared
using genomic DNA from blood cells obtained from a
woman, her daughter, and three men who all claim to be
the girl’s father.
MCF1 F2 F3
Based on the DNA proles, what can be determined
about paternity in this case?
ANS: Neither F1 nor F2 could be the girl’s biological father;
only individual F3 could be the child’s father.
16.13 Most forensic experts agree that proles of DNA from
blood samples obtained at crime scenes and on personal
items can provide convincing evidence for murder con-
victions. However, the defense attorneys sometimes
argue successfully that sloppiness in handling blood sam-
ples results in contamination of the samples. What prob-
lems would contamination of blood samples present in
the interpretation of DNA proles? Would you expect
such errors to lead to the conviction of an innocent per-
son or the acquittal of a guilty person?
ANS: Contamination of blood samples would introduce more
variability into DNA proles. This would lead to a lack
of allelic matching of proles obtained from the blood
samples and from the defendant. Mixing errors would be
expected to lead to the acquittal of a guilty person and
not to the conviction of an innocent person. Only the
mislabeling of samples could implicate someone who is
innocent.
16.14 The Ti plasmid contains a region referred to as T-DNA.
Why is this region called T-DNA, and what is its
signicance?
ANS: The T in T-DNA is an abbreviation for “transferred.”
The T-DNA region of the Ti plasmid is the segment
that is transferred from the Ti plasmid of the bacterium
to the chromosomes of the plant cells during Agrobacte-
rium tumefaciens-mediated transformation.
16.15 The generation of transgenic plants using A. tumefaciens-
mediated transformation often results in multiple sites of
insertion. These sites frequently vary in the level of
transgene expression. What approaches could you use to
determine whether or not transgenic plants carry more
than one transgene and, if so, where the transgenes are
inserted into chromosomes?
ANS: Probing Southern blots of restriction enzyme-digested
DNA of the transgenic plants with
32
P-labeled transgene
may provide evidence of multiple insertions but would
not reveal the genomic location of the inserts. Fluores-
cence in situ hybridization (FISH) is a powerful proce-
dure for determining the genomic location of gene
inserts. FISH is used to visualize the location of trans-
genes in chromosomes.
16.16 Disarmed retroviral vectors can be used to introduce
genes into higher animals including humans. What
advantages do retroviral vectors have over other kinds of
gene-transfer vectors? What disadvantages?
ANS: Disarmed retroviruses are lacking genes essential for
reproduction in host cells but can still integrate into the
DNA of the host cell in the proviral state. The retroviral
genomes are small enough to allow them to be manipu-
lated easily in vitro and yet will accept foreign DNA
inserts of average gene size. The retroviruses contain
strong promoters in their long terminal repeats that can
be used to drive high levels of transcription of the for-
eign gene insert.
16.17 Transgenic mice are now routinely produced and studied
in research laboratories throughout the world. How are
transgenic mice produced? What kinds of information
can be obtained from studies performed on transgenic
mice? Does this information have any importance to the
practice of medicine? If so, what?
ANS: Transgenic mice are usually produced by microinjecting
the genes of interest into pronuclei of fertilized eggs or
by infecting pre-implantation embryos with retroviral
vectors containing the genes of interest. Transgenic mice
provide invaluable tools for studies of gene expression,
mammalian development, and the immune system of
mammals. Transgenic mice are of major importance in
medicine; they provide the model system most closely
related to humans. They have been, and undoubtedly
will continue to be, of great value in developing the tools
and technology that will be used for human gene therapy
in the future.
16.18 Two men claim to be the father of baby Joyce Doe. Joyce’s
mother had her CODIS STR DNA prole analyzed and
was homozygous for allele 8 at the TPOX locus (allele 8
contains 8 repeats of the GAAT sequence at this poly-
morphic locus). Baby Joyce is heterozygous for alleles 8
and 11 at this locus. In an attempt to resolve the disputed
paternity, the two men were tested for their STR DNA
proles at the TPOX locus on chromosome 2. Putative
father 1 was heterozygous for alleles 8 and 11 at the
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 70 8/14/2015 6:43:02 PM

Answers to All Questions and Problems WC-71
TPOX locus, and putative father 2 was homozygous for
allele 11 at this locus. Can these results resolve this case
of disputed paternity. If so, who is the biological father? If
not, why not?
ANS: The results cannot resolve the case of disputed paternity.
Baby Joyce received allele 8 at the TPOX locus from her
mother. Given that baby Joyce is heterozygous for alleles
8 and 11 at the TPOX locus, she must have received
allele 11 from her father. However, both of the putative
fathers carry allele 11 and could have passed it on to baby
Joyce.
16.19 Many valuable human proteins contain carbohydrate or
lipid components that are added posttranslationally. Bac-
teria do not contain the enzymes needed to add these
components to primary translation products. How might
these proteins be produced using transgenic animals?
ANS: Posttranslationally modied proteins can be produced in
transgenic eukaryotic cells growing in culture or in
transgenic plants and animals. Indeed, transgenic sheep
have been produced that secrete human blood-clotting
factor IX and a1-antitrypsin in their milk. These sheep
were produced by fusing the coding sequences of the
respective genes to a DNA sequence that encodes the
signal peptide required for secretion and introducing
this chimeric gene into fertilized eggs that were then
implanted and allowed to develop into transgenic ani-
mals. In principle, this approach could be used to pro-
duce any protein of interest.
16.20 Richard Meagher and coworkers have cloned a family of
10 genes that encode actins (a major component of the
cytoskeleton) in Arabidopsis thaliana. The 10 actin gene
products are similar, often differing by just a few amino
acids. Thus, the coding sequences of the 10 genes are
also very similar, so that the coding region of one gene
will cross-hybridize with the coding regions of the other
nine genes. In contrast, the noncoding regions of the 10
genes are quite divergent. Meagher has hypothesized
that the 10 actin genes exhibit quite different temporal
and spatial patterns of expression. You have been hired
by Meagher to test this hypothesis. Design experiments
that will allow you to determine the temporal and spatial
pattern of expression of each of the 10 actin genes in
Arabidopsis.
ANS: You can subclone the 3′ noncoding regions (sequences
between the translation-termination codons and the 3′
termini of the transcripts) of the actin genes and use
these sequences as gene-specic hybridization probes,
after verifying their specicity (no cross-hybridization to
the other genes in the gene family). This procedure has
worked elegantly for the 15 tubulin genes and the 10
actin genes of Arabidopsis, because the transcript
sequences are very divergent in the 5′ and 3′ noncoding
regions. You cannot, of course, use intron sequences
because they are excised during pre-mRNA processing.
These 3′ noncoding gene-specic hybridization probes
are then used to measure individual gene transcript lev-
els in various organs and tissues of developing plants by
either northern blot or in situ hybridization experiments.
Alternatively, the 5′ and 3′ noncoding regions can be
used to design gene-specic PCR primers, and reverse
transcription PCR (RT-PCR) can be used to measure
individual gene transcript levels in organs and tissues.
Indeed, Meagher and colleagues have already used this
approach to document the striking temporal and tissue-
specic patterns of actin gene expression in Arabidopsis.
16.21 The rst transgenic mice resulted from microinjecting
fertilized eggs with vector DNA similar to that dia-
grammed in Figure 16.15 except that it contained a pro-
moter for the mammalian metallothionein gene linked to
the HGH gene. The resulting transgenic mice showed
elevated levels of HGH in tissues of organs other than the
pituitary gland—for example, in heart, lung, and liver—
and the pituitary gland underwent atrophy. How might
the production of HGH in transgenic animals be better
regulated, with expression restricted to the pituitary gland?
ANS: The vector described contains the HGH gene; however,
it does not contain a mammalian HGH promoter that
will regulate the expression of the transgene in the
appropriate tissues. Construction of vectors containing a
properly positioned mammalian HGH-promoter
sequence should result in transgenic mice in which
HGH synthesis is restricted to the pituitary gland.
16.22 How do the reverse genetic approaches used to dissect
biological processes differ from classical genetic
approaches?
ANS: Classical genetic approaches use mutational dissection to
probe the functions of genes. Mutant alleles that produce
altered phenotypes are identied and used to investigate
the functions of the wild-type alleles. Comparative
molecular analyses of mutant and wild-type organisms
sometimes allow researchers to determine the precise
function of a gene. Reverse genetic approaches use the
known nucleotide sequences of genes to design proce-
dures to either produce null mutations in them or inhibit
their expression. RNA interference protocols are used to
block the expression of specic genes. T-DNA and trans-
poson insertion protocols are used to produce null muta-
tions (to “knock out” the function) of specic genes.
Basically, in classical genetics, you start with mutant
alleles and hope to uncover the wild-type gene function;
in reverse genetics, you start with the wild-type gene and
generate mutant alleles.
16.23 How can RNAi gene silencing be used to determine the
function of genes?
ANS: RNAi involves the use of double-stranded RNAs, where
one strand is complementary to the mRNA and the
other strand is equivalent to the mRNA, to silence the
expression of target genes. RNAi makes use of the
RNA-induced silencing complex (RISC) to block gene
expression (see Figure 16.23).
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 71 8/14/2015 6:43:02 PM

72-WC Answers to All Questions and Problems
16.24 How do insertional mutagenesis approaches differ from
other reverse genetic approaches?
ANS: Insertional mutagenesis approaches produce mutant
alleles—usually null alleles, or “knockouts,”—by the
insertion of foreign DNA into genes. Other reverse
genetic approaches, for example, RNA interference,
inhibit the expression of the genes but leave them struc-
turally intact.
16.25 Insertional mutagenesis is a powerful tool in both plants
and animals. However, when performing large-scale
insertional mutagenesis, what major advantage do plants
have over animals?
ANS: Plants have an advantage over animals in that once inser-
tional mutations are induced they can be stored for long
periods of time and distributed to researchers as dormant
seeds.
16.26 We discussed the unfortunate effects of insertional muta-
genesis in the four boys who developed leukemia after
treatment of X-linked severe combined immunode-
ciency disease by gene therapy. How might this conse-
quence of gene therapy be avoided in the future? Do you
believe that the use of somatic-cell gene therapy to treat
human diseases can ever be made 100 percent risk-free?
Why? Why not?
ANS: The vectors used in somatic-cell gene therapy must not
insert themselves preferentially into important genes,
especially proto-oncogenes. Ideally, the vectors should
insert themselves into unessential regions of the human
genome. However, such vectors may not exist. At the
minimum, however, vectors should be used that insert
into the genome at random sites so that their chance of
inserting into an important regulatory gene such as a
proto-oncogene is very low. Gene therapy will probably
never be completely risk-free, because anomalous inser-
tion events will always occur at some low frequency. No
biological process is 100 percent accurate. However, the
potential benets of gene therapy must outweigh the
potential risks before the procedure will become an
accepted tool for treating inherited diseases.
16.27 One strand of a gene in Arabidopsis thaliana has the fol-
lowing nucleotide sequence:
atgagtgacgggaggaggaagaagagcgtgaacggaggt
gcaccggcgcaaacaatcttggatgatcggagatctagtcttccgga
agttgaagcttctccaccggctgggaaacgagctgttatcaagagtgc
cgatatgaaagatgatatgcaaaaggaagctatcgaaatcgccatct
ccgcgtttgagaagtacagtgtggagaaggatatagctgagaatataa
agaaggagtttgacaagaaacatggtgctacttggcattgcattgttgg
tcgcaactttggttcttatgtaacgcatgagacaaaccatttcgtttacttct
acctcgaccagaaagctgtgctgctcttcaagtcgggttaa
The function(s) of this gene is still uncertain. (a) How
might insertional mutagenesis be used to investigate the
function(s) of the gene? (b) Design an experiment using
RNA interference to probe the function(s) of the gene.
ANS: (a) You would rst want to check the Salk Institute’s
Genome Analysis Laboratory web site to see if a T-DNA
or transposon insertion has already been identied in
this gene (see Problem 16.28). If so, you can simply order
seeds of the transgenic line from the Arabidopsis Biologi-
cal Resource Center at Ohio State University. If no
insertion is available in the gene, you can determine
where it maps in the genome and use transposons that
preferentially jump to nearby sites to identify a new
insertional mutation (see http://www.arabidopsis.org/
abrc/ima/jsp). (b) You can construct a gene that has sense
and antisense sequences transcribed to a single mRNA
molecule (see Figure 16.23b), introduce it into Arabidop-
sis plants by A. tumefaciens-mediated transformation, and
study its effect(s) on the expression of the gene and the
phenotype of transgenic plants. The transcript will form
a partially base-paired hairpin that will enter the RISC
silencing pathway and block the expression of the gene
(see Figure 16.23b).
16.28 Let’s check the Salk Institute’s Genome Analysis Labora-
tory web site (http://signal.salk.edu/cgi-bin/tdnaexpress)
to see if any of their T-DNA lines have insertions in the
gene shown in the previous question. At the SIGnAL
web site, scroll down to “Blast” and paste or type the
sequence in the box. The resulting map will show the
location of mapped T-DNA insertions relative to
the location of the gene (green rectangle at the top). The
blue arrows at the top right will let you focus on just the
short region containing the gene or relatively long
regions of chromosome 4 of Arabidopsis. Are there any
T-DNA insertions in the gene in question? Near the
gene?
ANS: A search of the Salk Institute’s Genome Analysis Labora-
tory (SIGnAL) web site reveals that there are numerous
T-DNA insertions (Salk T-DNA insertions and several
others) located in the promoter region of this gene.
Moreover, there are two insertion lines (FLAG_177C02
and FLAG_216D01) in the collection at the Institute of
Agronomic Research in Versailles, France, with inserts in
the coding region of this gene. All of these insertion lines
in these collections are available to researchers on
request. Thus, the function of this gene can be studied by
using these insertion lines.
16.29 The CRISPR/Cas9 anti-phage immunity system in Strep-
tococcus pyogenes deploys a variety of crRNAs derived from
the spacer and repeat sequences in the CRISPR array in
the S. pyogenes genome. In combination with the transac-
tivating RNA (tracrRNA), these crRNAs guide the Cas9
endonuclease to complementary sequences in infecting
phage genomes, whereupon Cas9 cleaves the phage DNA.
A requirement for cleavage is that the targeted phage
DNA sequence be immediately upstream of a protospacer
adjacent motif (PAM), which in the S. pyogenes system is
5′-NGG-3′. Why is it important that the CRISPR array
in the S. pyogenes genome not contain this PAM?
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 72 8/14/2015 6:43:02 PM

Answers to All Questions and Problems WC-73
ANS: It is important that the CRISPR array in the S. pyogenes
genome does not contain the PAM 5′NGG-3′ because if
it did, crRNAs generated from the array could target the
Cas9 endonuclease to the array and cleave it, resulting in
breakage of the S. pyogenes chromosome.
16.30 The Streptococcus pyogenes Cas9 endonuclease can be tar-
geted to a specic genomic DNA sequence by an sgRNA
that at its 5′ end has 20 nucleotides complementary to the
target sequence. If this target sequence is immediately
upstream of the protospacer adjacent motif (PAM)
5′NGG-3′, Cas9 will cleave the target DNA. Suppose you
have chosen a 20-nucleotide target sequence in the genome
of Drosophila melanogaster and that this sequence is next to
the required PAM. How could you determine if Cas9 will
cleave only this sequence in the Drosophila genome?
ANS: You could use the 20-nucleotide sequence as a query in
BLAST to scan the sequenced portion of the Drosophila
genome to see if all or part of the target sequence is pres-
ent anywhere else. If this sequence is present elsewhere,
then you could check to see if the PAM 5′-NGG-3′ is
immediately downstream of the sequence. If it is, then
the Cas9 endonuclease will cleave at this site as well as at
the intended target site.
16.31 How could the CRISPR/Cas9 system be used to create a
translocation between two autosomes in cultured human
cells?
ANS: Create two sgRNAs, one to target a sequence on a par-
ticular autosome and the other to target a sequence
on a different autosome. Then introduce these sgRNAs
and the Cas9 endonuclease into cultured cells to induce
breakage at the two target sites. The broken DNA mol-
ecules may be repaired by the NHEJ pathway, and if they
are, the broken pieces of different autosomes could be
joined covalently, creating a reciprocal translocation.
Chapter 17
17.1 How can inducible and repressible enzymes of microor-
ganisms be distinguished?
ANS: By studying the synthesis or lack of synthesis of the
enzyme in cells grown on chemically dened media. If
the enzyme is synthesized only in the presence of a cer-
tain metabolite or a particular set of metabolites, it is
probably inducible. If it is synthesized in the absence but
not in the presence of a particular metabolite or group of
metabolites, it is probably repressible.
17.2 Distinguish between (a) repression and (b) feedback
inhibition caused by the end-product of a biosynthetic
pathway. How do these two regulatory phenomena com-
plement each other to provide for the efcient regula-
tion of metabolism?
ANS: Repression occurs at the level of transcription during
enzyme synthesis. The end-product, or a derivative of
the end-product, of a repressible system acts as an
effector molecule that usually, if not always, combines
with the product of one or more regulator genes to turn
off the synthesis of the enzymes in the biosynthetic path-
way. Feedback inhibition occurs at the level of enzyme
activity; it usually involved the rst enzyme of the bio-
synthetic pathway. Feedback inhibition thus brings about
an immediate arrest of the biosynthesis of the end-
product. All together, feedback inhibition and repression
rapidly and efciently turn off the synthesis of both the
enzymes and the end-products that no longer need to be
synthesized by the cell.
17.3 In the lactose operon of E. coli, what is the function of
each of the following genes or sites: (a) regulator, (b)
operator, (c) promoter, (d) structural gene Z, and (e)
structural gene Y?
ANS:
Gene or Regulatory
Element Function
(a) Regulator gene Encodes the repressor
(b) Operator Binding site of repressor
(c) Promoter Binding site of RNA
polymerase and CAP-cAMP
complex
(d) Structural gene Z Encodes b-galactosidase
(e) Structural gene Y Encodes b-galactoside
permease
17.4 What would be the result of inactivation by mutation of
the following genes or sites in the E. coli lactose operon:
(a) regulator, (b) operator, (c) promoter, (d) structural
gene Z, and (e) structural gene Y?
ANS: (a) Constitutive synthesis of the lac enzymes.
(b) Constitutive synthesis of the lac enzymes.
(c) Uninducibility of the lac enzymes.
(d) No b-galactosidase activity.
(e) No b-galactoside permease activity.
17.5 Groups of alleles associated with the lactose operon are
as follows (in order of dominance for each allelic series):
repressor, I
s
(superrepressor), I
(inducible), and I
(con-
stitutive); operator, O
c
(constitutive, cis
dominant) and
O
(inducible, cis-dominant); structural, Z
and Y
. (a)
Which of the following genotypes will produce b-galac-
tosidase and b-galactoside permease if lactose is present:
(1) I
O
Z
Y
, (2) I
O
c
Z
Y
, (3) I
s
O
c
Z
Y
, (4) I
s
O
Z
Y
,
and (5) I
O
Z
Y
? (b) Which of the above genotypes
will produce b-galactosidase and b-galactoside permease
if lactose is absent? Why?
ANS: (a) 1, 2, 3, and 5. Genotype 1 is wild-type and inducible,
whereas genotypes 2, 3, and 5 are constitutive; all except 4
will produce b-galactosidase and b-galactoside permease in
the presence of lactose. However, genotype 4 has a super-
repressor mutation (I
s
) and is uninducible with normal
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 73 8/14/2015 6:43:02 PM

74-WC Answers to All Questions and Problems
levels of lactose. (b) 2, 3, and 5. In genotypes 2 and 3, the
repressor cannot bind to O
c
and in genotype 5, no repressor
is made; both situations render the operon constitutive.
17.6 Assume that you have discovered a new strain of E. coli
that has a mutation in the lac operator region that causes
the wild-type repressor protein to bind irreversibly
to the operator. You have named this operator mutant
O
sb
for “superbinding” operator. (a) What phenotype
would a partial diploid of genotype I
O
sb
Z
Y
/I
O
Z
Y
have with respect to the synthesis of the enzymes
b-galactosidase and b-galactoside permease? (b) Does
your new O
sb
mutation exhibit cis or trans dominance in
its effects on the regulation of the lac operon?
ANS: (a) b-Galactosidase will be produced only when lactose is
present. Permease will not be produced at all. (b) cis
dominance.
17.7 Why is the O
c
mutation in the E. coli lac operon epistatic
to the I
s
mutation?
ANS: The O
c
mutant prevents the repressor from binding to
the operator. The I
s
mutant repressor cannot bind to O
c
.
The I
s
mutant protein has a defect in the allosteric site
that binds allolactose but has a normal operator binding
site. Therefore, because the single O
c
mutant would have
the same phenotype as the O
c
I
s
double mutant, the O
c
mutation is, by denition, epistatic to I
s
.
17.8 For each of the following partial diploids indicate
whether enzyme synthesis is constitutive or inducible
(see Problem 17.5 for dominance relationships):
(a) I
O
Z
Y
/I
O
Z
Y
, (b) I
O
Z
Y
/I
O
c
Z
Y
,
(c) I
O
c
Z
Y
/I
O
c
Z
Y
, (d) I
O
Z
Y
/IO
Z
Y
,
(e) I
O
Z
Y
/IO
Z
Y
.
Why?
ANS: (a) Inducible, this is the wild-type genotype and
phenotype.
(b) Constitutive, the O
c
mutation produces an operator
that is not recognized by the lac repressor.
(c) Constitutive, same as for (b).
(d) Inducible, I
is dominant to I
.
(e) Constitutive, no active repressor is synthesized in this
bacterium.
17.9 Write the partial diploid genotype for a strain that will
(a) produce b-galactosidase constitutively and permease
inducibly and (b) produce b-galactosidase constitutively
but not permease either constitutively or inducibly, even
though a Y
gene is known to be present.
ANS: (a) I
O
c
Z
Y
/I
O
Z
Y
(b) I
O
c
A
Y
/I
s
O
Z
Y
17.10 As a genetics historian, you are repeating some of the
classic experiments conducted by Jacob and Monod with
the lactose operon in E. coli. You use an F plasmid to
construct several E. coli strains that are partially diploid
for the lac operon. You construct strains with the follow-
ing genotypes: (1) I
O
c
Z
Y
/I
O
Z
Y
, (2) I
O
c
Z
Y
/
I
O
Z
Y
, (3) I
O
Z
Y
/I
O
Z
Y
, (4) I
s
O
Z
Y
/
I
O
Z
Y
, and (5) I
O
c
Z
Y
/I
s
O
Z
Y
. (a) Which of
these strains will produce functional b-galactosidase in
both the presence and absence of lactose? (b) Which of
these strains will exhibit constitutive synthesis of func-
tional b-galactoside permease? (c) Which of these strains
will express both gene Z and gene Y constitutively and
will produce functional products (b-galactosidase and
b-galactoside permease) of both genes? (d) Which of
these strains will show cis dominance of lac operon regu-
latory elements? (e) Which of these strains will exhibit
trans dominance of lac operon regulatory elements?
ANS: (a) 1, 5.
(b) 2, 5.
(c) 5.
(d) 1, 2, 5.
(e) 3, 4.
17.11 Constitutive mutations produce elevated enzyme levels
at all times; they may be of two types: O
c
or I
. Assume
that all other DNA present is wild-type. Outline how the
two constitutive mutants can be distinguished with
respect to (a) map position, (b) regulation of enzyme
levels in O
c
/O
versus I
/I
partial diploids, and (c) the
position of the structural genes affected by an O
c
muta-
tion versus the genes affected by an I
mutation in a par-
tial diploid.
ANS: (a) The O
c
mutations map very close to the Z structural
gene; I
−
mutations map slightly farther from the struc-
tural gene (but still very close by; see Figure 17.5).
(b) An I
O
Z
Y
/I
O
c
Z
Y
partial diploid would exhibit
constitutive synthesis of b-galactosidase and b-galacto-
side permease, whereas an I
O
Z
Y
/I
O
Z
Y
partial
diploid would be inducible for the synthesis of these
enzymes.
(c) The O
c
mutation is cis-dominant; the I
mutation is
trans-recessive.
17.12 How could the tryptophan operon in E. coli have devel-
oped and been maintained by evolution?
ANS: The system could have developed from a series of tan-
dem duplications of a single ancestral gene. Mutational
changes that make the system more efcient and, there-
fore, favored by natural selection could have brought the
system to its present level of efciency.
17.13 Of what biological signicance is the phenomenon of
catabolite repression?
ANS: Catabolite repression has apparently evolved to assure
the use of glucose as a carbon source when this carbohy-
drate is available, rather than less efcient energy sources.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 74 8/14/2015 6:43:03 PM

Answers to All Questions and Problems WC-75
17.14 How might the concentration of glucose in the medium
in which an E. coli cell is growing regulate the intracel-
lular level of cyclic AMP?
ANS: Possibly by directly or indirectly inhibiting the enzyme
adenylcyclase, which catalyzes the synthesis of cyclic
AMP from ATP.
17.15 Is the CAP–cAMP effect on the transcription of the lac
operon an example of positive or negative regulation? Why?
ANS: Positive regulation; the CAP–cAMP complex has a posi-
tive effect on the expression of the lac operon. It func-
tions in turning on the transcription of the structural
genes in the operon.
17.16 Would it be possible to isolate E. coli mutants in which
the transcription of the lac operon is not sensitive to
catabolite repression? If so, in what genes might the
mutations be located?
ANS: Yes; in the gene encoding CAP. Some mutations in this
gene might result in a CAP that binds to the promoter in
the absence of cAMP. Also, mutations in the gene (or
genes) coding for the protein (or proteins) that regulate
the cAMP level as a function of glucose concentration.
17.17 Using examples, distinguish between negative regulatory
mechanisms and positive regulatory mechanisms.
ANS: Negative regulatory mechanisms, such as that involving
the repressor in the lactose operon, block the transcrip-
tion of the structural genes of the operon, whereas posi-
tive mechanisms, such as the CAP–cAMP complex in the
lac operon, promote the transcription of the structural
genes of the operon.
17.18 The following table gives the relative activities of the
enzymes b-galactosidase and b-galactoside permease in
cells with different genotypes at the lac locus in E. coli.
The level of activity of each enzyme in wild-type E. coli
not carrying F’s was arbitrarily set at 100; all other values
are relative to the observed levels of activity in these
wild-type bacteria. Based on the data given in the table
for genotypes 1 through 4, ll in the levels of enzyme
activity that would be expected for the fth genotype.
-Galactoside
-Galactosidase
Permease
Genotype - Inducer Inducer - Inducer Inducer
1. I
O
Z
Y
0.1 100 0.1 100
2. I
O
Z
Y
100 100 100 100
3. I
O
c
Z
Y
25 100 25 100
4. I
O
Z
Y
/
F I
O
Z
Y
200 200 100 100
5. I
O
c
Z
Y
/
F I
O
Z
Y
ANS: 0.1; 100; 25.1; 200.
17.19 The rate of transcription of the trp operon in E. coli
is controlled by both (1) repression/derepression and
(2) attenuation. By what mechanisms do these two regu-
latory processes modulate trp operon transcript levels?
ANS: Repression/derepression of the trp operon occurs at the
level of transcription initiation, modulating the fre-
quency at which RNA polymerase initiates transcription
from the trp operon promoters. Attenuation modulates
trp transcript levels by altering the frequency of termina-
tion of transcription within the trp operon leader region
(trpL).
17.20 What effect will deletion of the trpL region of the trp
operon have on the rates of synthesis of the enzymes
encoded by the ve genes in the trp operon in E. coli cells
growing in the presence of tryptophan?
ANS: Deletion of the trpL region would result in the levels of
the tryptophan biosynthetic enzymes in cells growing in
the presence of tryptophan being increased about 10-fold
because attenuation would no longer occur if this region
were absent.
17.21 By what mechanism does the presence of tryptophan in
the medium in which E. coli cells are growing result in
premature termination or attenuation of transcription of
the trp operon?
ANS: First, remember that transcription and translation are
coupled in prokaryotes. When tryptophan is present in
cells, tryptophan-charged tRNA
Trp
is produced. This
allows translation of the trp leader sequence through the
two UGG Trp codons to the trp leader sequence UGA
termination codon. This translation of the trp leader
region prevents base-pairing between the partially com-
plementary mRNA leader sequences 75–83 and 110–121
(see Figure 17.15b), which in turn permits formation of
the transcription–termination “hairpin” involving leader
sequences 110–121 and 126–134 (see Figure 17.15c).
17.22 Suppose that you used site-specic mutagenesis to mod-
ify the trpL sequence such that the two UGG Trp codons
at positions 54–56 and 57–60 (see Figure 17.14) in the
mRNA leader sequence were changed to GGG Gly
codons. Will attenuation of the trp operon still be regu-
lated by the presence or absence of tryptophan in the
medium in which the E. coli cells are growing?
ANS: No. Attenuation of the trp operon would now be con-
trolled by the presence or absence of Gly-tRNA
Gly
.
17.23 What do trp attenuation and the lysine riboswitch have
in common?
ANS: Both trp attenuation and the lysine riboswitch turn off
gene expression by terminating transcription upstream
from the coding regions of the regulated genes. Both
involve the formation of alternative mRNA secondary
structures—switching between the formation of antiter-
minator and transcription–terminator hairpins—in
response to the presence or absence of a specic
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 75 8/14/2015 6:43:03 PM

76-WC Answers to All Questions and Problems
metabolite (compare Figure 17.15 and Figure 2 in the
Focus On The Lysine Riboswitch).
17.24 Would attenuation of the type that regulates the level of
trp transcripts in E. coli be likely to occur in eukaryotic
organisms?
ANS: No. Since transcription (nucleus) and translation (cyto-
plasm) are no coupled in eukaryotes, attenuation of the
type occurring in prokaryotes would not be possible.
Chapter 18
18.1 Operons are common in bacteria but not in eukaryotes.
Suggest a reason why.
ANS: In multicellular eukaryotes, the environment of an indi-
vidual cell is relatively stable. There is no need to respond
quickly to changes in the external environment. In addi-
tion, the development of a multicellular organism
involves complex regulatory hierarchies composed of
hundreds of different genes. The expression of these
genes is regulated spatially and temporally, often through
intricate intercellular signaling processes.
18.2 In bacteria, translation of an mRNA begins before the
synthesis of that mRNA is completed. Why is this “cou-
pling” of transcription and translation not possible in
eukaryotes?
ANS: Coupling of transcription and translation is not possible
in eukaryotes because these two processes take place in
different cellular compartments—transcription in the
nucleus and translation in the cytoplasm.
18.3 Muscular dystrophy in humans is caused by mutations in
an X-linked gene that encodes a protein called dystro-
phin. What techniques could you use to determine if this
gene is active in different types of cells, say skin cells,
nerve cells, and muscle cells?
ANS: Activity of the dystrophin gene could be assessed by blot-
ting RNA extracted from the different types of cells and
hybridizing it with a probe from the gene (northern
blotting); or the RNA could be reverse transcribed into
cDNA using one or more primers specic to the dystro-
phin gene and the resulting cDNA could be amplied by
the polymerase chain reaction (RT-PCR). Another tech-
nique would be to hybridize dystrophin RNA in situ—that
is, in the cells themselves—with a probe from the gene.
It would also be possible to check each cell type for pro-
duction of dystrophin protein by using anti-dystrophin
antibodies to analyze proteins from the different cell
types on western blots or analyze the proteins in the cells
themselves—that is, in situ.
18.4 Why do steroid hormones interact with receptors inside
the cell, whereas peptide hormones interact with recep-
tors on the cell surface?
ANS: Steroid hormones are small, lipid-soluble molecules that
have little difculty passing through the cell membrane.
Peptide hormones are typically too large to pass through
the cell membrane freely; rather, their inuence must be
mediated by a signaling system that begins with a mem-
brane-bound receptor protein that binds the hormone.
18.5 In the polytene chromosomes of Drosophila larvae (Chap-
ter 6), some bands form large “puffs” when the larvae are
subjected to high temperatures. How could you show
that these puffs contain genes that are vigorously tran-
scribed in response to this heat shock treatment?
ANS: One procedure would be to provide larvae with radioac-
tively labeled UTP, a building block of RNA, under dif-
ferent conditions—with and without heat shock. Then
prepare samples of polytene cells from these larvae for
autoradiography. If the heat shock-induced puffs contain
genes that are vigorously transcribed, the radioactive sig-
nal should be abundant in the puffs.
18.6 How would you distinguish between an enhancer and a
promoter?
ANS: An enhancer can be located upstream, downstream, or
within a gene, and it functions independently of its ori-
entation. A promoter is almost always immediately
upstream of a gene and it functions only in one direction
with respect to the gene.
18.7 Tropomyosins are proteins that mediate the interaction
of actin and troponin, two proteins involved in muscle
contractions. In higher animals, tropomyosins exist as a
family of closely related proteins that share some amino
acid sequences but differ in others. Explain how these
proteins could be created from the transcript of a single
gene.
ANS: By alternate splicing of the transcript.
18.8 A polypeptide consists of three separate segments of
amino acids, A—B—C. Another polypeptide contains
segments A and C but not segment B. How might you
determine if these two polypeptides are produced by
translating alternately spliced versions of RNA from a
single gene or by translating mRNA from two different
genes?
ANS: Southern blotting of genomic DNA digested with an
appropriate restriction enzyme, followed by hybridiza-
tion of the blot with a probe containing the DNA encod-
ing segments A and B, or B and C, or at least parts of
these adjacent segments. If one DNA fragment is
detected on the blot, the two polypeptides are encoded
by a single gene whose RNA is alternately spliced to pro-
duce two mRNAs. If two DNA fragments are detected,
the two polypeptides are encoded by two different genes.
18.9 What techniques could be used to show that a plant gene
is transcribed when the plant is illuminated with light?
ANS: Northern blotting of RNA extracted from plants grown
with and without light, or PCR amplication of cDNA
made by reverse transcribing these same RNA extracts.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 76 8/14/2015 6:43:03 PM

Answers to All Questions and Problems WC-77
18.10 When introns were rst discovered, they were thought
to be genetic “junk”—that is, sequences without any use-
ful function. In fact, they appeared to be worse than junk
because they actually interrupted the coding sequences
of genes. However, among eukaryotes, introns are perva-
sive and anything that is pervasive in biology usually has
a function. What function might introns have? What
benet might they confer on an organism?
ANS: Introns make it possible for genes to encode different—
but related—polypeptides by alternate splicing of their
RNA transcripts.
18.11 The GAL4 transcription factor in yeast regulates two
adjacent genes, GAL1 and GAL10, by binding to DNA
sequences between them. These two genes are tran-
scribed in opposite directions on the chromosome, one
to the left of the GAL4 protein’s binding site and the
other to the right of this site. What property of enhanc-
ers does this situation illustrate?
ANS: That enhancers can function in either orientation.
18.12 Using the techniques of genetic engineering, a researcher
has constructed a fusion gene containing the heat-shock
response elements from a Drosophila hsp70 gene and the
coding region of a jellysh gene (gfp) for green uores-
cent protein. This fusion gene has been inserted into the
chromosomes of living Drosophila by the technique of
transposon-mediated transformation (Chapter 21 on the
Instructor Companion site). Under what conditions will
the green uorescent protein be synthesized in these
genetically transformed ies? Explain.
ANS: The green uorescent protein will be made after the ies
are heat shocked.
18.13 Suppose that the segment of the hsp70 gene that was used
to make the hsp70/gfp fusion in the preceding problem
had mutations in each of its heat-shock response ele-
ments. Would the green uorescent protein encoded by
this fusion gene be synthesized in genetically trans-
formed ies?
ANS: Probably not unless the promoter of the gfp gene is recog-
nized and transcribed by the Drosophila RNA polymerase
independently of the heat-shock response elements.
18.14 The polypeptide products of two different genes, A and
B, each function as transcription factors. These polypep-
tides interact to form dimers: AA homodimers, BB
homodimers, and AB heterodimers. If the A and B poly-
peptides are equally abundant in cells, and if dimer for-
mation is random, what is the expected ratio of
homodimers to heterodimers in these cells?
ANS: With equal abundance of the A and B polypeptides, AA
homodimers should constitute 1/4 of the total dimers
formed, BB homodimers should constitute 1/4 of the
total, and AB heterodimers should constitute 1/2 of the
total. The expected ratio of homodimers to heterodimers
is therefore (1/4 1/4):(1/2) 1:1.
18.15 A particular transcription factor binds to enhancers in 40
different genes. Predict the phenotype of individuals homo-
zygous for a frameshift mutation in the coding sequence of
the gene that species this transcription factor.
ANS: The mutation is likely to be lethal in homozygous condi-
tion because the transcription factor controls so many
different genes, and a frameshift mutation in the coding
sequence will almost certainly destroy the transcription
factor’s function.
18.16 The alternately spliced forms of the RNA from the Dro-
sophila doublesex gene encode proteins that are needed to
block the development of one or the other set of sexual
characteristics. The protein that is made in female ani-
mals blocks the development of male characteristics, and
the protein that is made in male animals blocks the
development of female characteristics. Predict the phe-
notype of XX and XY animals homozygous for a null
mutation in the doublesex gene.
ANS: Both XX and XY animals would develop as intersexes
because neither of the forms of the doublesex protein
will be able to block sexual development. In these ani-
mals, both developmental pathways will be carried out,
leading to tissues that have both male and female
characteristics.
18.17 The RNA from the Drosophila Sex-lethal (Sxl) gene is
alternately spliced. In males, the sequence of the mRNA
derived from the primary transcript contains all eight
exons of the Sxl gene. In females, the mRNA contains
only seven of the exons because during splicing exon 3 is
removed from the primary transcript along with its
anking introns. The coding region in the female’s
mRNA is therefore shorter than it is in the male’s mRNA.
However, the protein encoded by the female’s mRNA is
longer than the one encoded by the male’s mRNA. How
might you explain this paradox?
ANS: Exon 3 contains an in-frame stop codon. Thus, the pro-
tein translated from the Sxl mRNA in males will be
shorter than the protein translated from the shorter Sxl
mRNA in females.
18.18 In Drosophila, expression of the yellow gene is needed for
the formation of dark pigment in many different tissues;
without this expression, a tissue appears yellow in color.
In the wings, the expression of the yellow gene is con-
trolled by an enhancer located upstream of the gene’s
transcription initiation site. In the tarsal claws, expres-
sion is controlled by an enhancer located within the
gene’s only intron. Suppose that by genetic engineering,
the wing enhancer is placed within the intron and the
claw enhancer is placed upstream of the transcription
initiation site. Would a y that carried this modied yel-
low gene in place of its natural yellow gene have darkly
pigmented wings and claws? Explain.
ANS: Yes. Enhancers are able to function in different positions
in and around a gene.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 77 8/14/2015 6:43:03 PM

78-WC Answers to All Questions and Problems
18.19 A researcher suspects that a 550-bp-long intron contains
an enhancer that drives expression of an Arabidopsis gene
specically in root tip tissue. Outline an experiment to
test this hypothesis.
ANS: The intron could be placed in a GUS expression vector,
which could then be inserted into Arabidopsis plants. If
the intron contains an enhancer that drives gene expres-
sion in root tips, transgenic plants should show GUS
expression in their root tips. See the Problem-Solving
Skills feature in Chapter 18 for an example of this type of
analysis.
18.20 What is the nature of each of the following classes of
enzymes? What does each type of enzyme do to chroma-
tin? (a) HATs, (b) HDACs, (c) HMTs.
ANS: (a) HATs, histone acetyl transferases, transfer acetyl
groups to the amino acid lysine in histones; (b) HDACs,
histone deacetylases, remove acetyl groups from these
lysines; (c) HMTs, histone methyl transferases, transfer
methyl groups to lysine, arginine, and histidine in
histones.
18.21 In Drosophila larvae, the single X chromosome in males
appears diffuse and bloated in the polytene cells of the
salivary gland. Is this observation compatible with the
idea that X-linked genes are hyperactivated in Drosophila
males?
ANS: Yes. The diffuse, bloated appearance indicates that the
genes on this chromosome are being transcribed
vigorously—the chromatin is “open for business.”
18.22 Suppose that the LCR of the b-globin gene cluster was
deleted from one of the two chromosomes 11 in a man.
What disease might this deletion cause?
ANS: The LCR regulates the expression of all the genes linked
to it. Deletion of the LCR would ablate or impair globin
gene expression from one of the two chromosomes 11.
With less b-globin being produced, the individual would
likely suffer from anemia.
18.23 Would double-stranded RNA derived from an intron be
able to induce RNA interference?
ANS: Short interfering RNAs target messenger RNA mole-
cules, which are devoid of introns. Thus, if siRNA were
made from double-stranded RNA derived from an
intron, it would be ineffective against an mRNA target.
18.24 An RNA interference-like phenomenon has been impli-
cated in the regulation of transposable elements. In Dro-
sophila, two of the key proteins involved in this regulation
are encoded by the genes aubergine and piwi. Flies that
are homozygous for mutant alleles of these genes are
lethal or sterile, but ies that are heterozygous for them
are viable and fertile. Suppose that you have strains of
Drosophila that are heterozygous for aubergine or piwi
mutant alleles. Why might the genomic mutation rate in
these mutant strains be greater than the genomic muta-
tion rate in a wild-type strain?
ANS: The aubergine and piwi gene products mediate the RNAi-
like response. Reduction in the amount of aubergine or
piwi protein would likely impair the organism’s ability to
mount this response, and without a vigorous capacity for
regulation, transposable elements would be more likely
to move in the genome. This movement would likely
cause mutations because the transposons could insert
into genes and inactivate them. Thus, Drosophila that are
heterozygous for mutations in aubergine or piwi might
experience higher mutation rates than Drosophila lacking
these mutations.
18.25 Suppose that female mice homozygous for the a allele of
the Igf2 gene are crossed to male mice homozygous for
the b allele of this gene. Which of these two alleles will
be expressed in the F
1
progeny?
ANS: The paternally contributed allele (b) will be expressed in
the F
1
progeny.
18.26 Epigenetic states are transmitted clonally through cell
division. What kinds of observations indicate that these
states can be reversed or reset?
ANS: Here are two observations that show reversal or resetting
of an epigenetic state: (1) For imprinted genes in mam-
mals, the epigenetic state can be reset when the gene
passes through the germ line of the opposite sex.
(2) Genes on the inactive X chromosome in mammals
are reactivated in the female germ line.
18.27 A researcher hypothesizes that in mice gene A is actively
transcribed in liver cells, whereas gene B is actively tran-
scribed in brain cells. Describe procedures that would
allow the researcher to test this hypothesis.
ANS: RNA could be isolated from liver and brain tissue.
Northern blotting or RT-PCR with this RNA could
then establish which of the genes (A or B) is transcribed
in which tissue. For northern blotting, the RNA samples
would be fractionated in a denaturing gel and blotted to
a membrane and then the RNA on the membrane would
be hybridized with gene-specic probes, rst for one
gene, then for the other (or the researcher could prepare
two separate blots and hybridize each one with a differ-
ent probe). For RT-PCR, the RNA samples would be
reverse transcribed into cDNA using primers specic for
each gene; then the cDNA molecules would be amplied
by standard PCR and the products of the amplications
would be fractionated by gel electrophoresis to deter-
mine which gene’s RNA was present in the original
samples.
18.28 Suppose that the hypothesis mentioned in the previous
question is correct and that gene A is actively transcribed
in liver cells, whereas gene B is actively transcribed in
brain cells. The researcher now extracts equivalent
amounts of chromatin from liver and brain tissues and
treats these extracts separately with DNase I for a lim-
ited period of time. If the DNA that remains after the
treatments is then fractionated by gel electrophoresis,
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 78 8/14/2015 6:43:03 PM

Answers to All Questions and Problems WC-79
transferred to a membrane by Southern blotting, and
hybridized with a radioactively labeled probe specic for
gene A, which sample (liver or brain) will be expected to
show the greater signal on the autoradiogram? Explain
your answer.
ANS: The sample of chromatin from brain tissue would be
expected to show the greater signal on a Southern blot
hybridized with a probe specic for gene A. The reason
is that this gene is not so well transcribed in brain cells;
consequently, it will be more resistant to digestion with
DNase I in chromatin derived from brain cells than in
chromatin derived from liver cells in which it is actively
transcribed (and therefore more open to digestion with
DNase I).
18.29 Why do null mutations in the msl gene in Drosophila have
no effect in females?
ANS: The msl gene is not functional in females.
18.30 Suppose that a woman carries an X chromosome in
which the XIST locus has been deleted. The woman’s
other X chromosome has an intact XIST locus. What
pattern of X-inactivation would be observed throughout
the woman’s body?
ANS: The X chromosome containing the intact XIST locus
will be silenced in all cases because that locus is located
with the X inactivation center (XIC) and aids in silencing
the inactive X.
18.31 In Drosophila, the variegated phenotype of the white mot-
tled allele is suppressed by a dominant autosomal muta-
tion that knocks out the function of the gene for
heterochromatin protein 1 (HP1), an important factor in
heterochromatin formation. Flies with the white mottled
allele and the suppressor mutation have an almost uni-
form red color in their eyes; without the suppressor
mutation, the eyes are mosaics of red and white tissue.
Can you suggest an explanation for the effect of the sup-
pressor mutation?
ANS: HP1, the protein encoded by the wild-type allele of the
suppressor gene, is involved in chromatin organization.
Perhaps this heterochromatic protein spreads from the
region near the inversion breakpoint in the chromosome
that carries the white mottled allele and brings about the
“heterochromatization” of the white locus. When HP1 is
depleted by knocking out one copy of the gene encoding
it—that is, by putting the suppressor mutation into the
y’s genotype, the “heterochromatization” of the white
locus would be less likely to occur, and perhaps not occur
at all. The white locus would then function fully in all
eye cells, producing a uniform red eye color.
18.32 The sheep Dolly (Chapter 2) was the rst cloned mam-
mal. Dolly was created by implanting a nucleus from a
cell taken from the udder of a female sheep into an enu-
cleated egg. This nucleus had two X chromosomes, and
because it came from a differentiated cell, one of them
must have been inactivated. If the udder cell was
heterozygous for at least one X-linked gene whose
expression you could assay, how could you determine if
all of Dolly’s cells had the same X chromosome inacti-
vated? If, upon testing, Dolly’s cells prove to be mosaic
for X chromosome activity—that is, different X’s are
active in different clones of cells—what must have hap-
pened during her embryological development?
ANS: Take samples of cells from Dolly and determine if the
products of both alleles of the X-linked gene are present
in them. If the products of both alleles are present, then
Dolly must be a genetic mosaic for X chromosome
activity. Thus, during her development, the pattern of
X-inactivation that existed in the udder cell from which
she was derived must have been reset. If only one of the
gene’s products is detected—and if the sample of cells is
representative of all Dolly’s cells—then Dolly must have
maintained the pattern of X-inactivation that existed in
the udder cell from which she was derived.
Chapter 19
19.1 If heart disease is considered to be a threshold trait, what
genetic and environmental factors might contribute to
the underlying liability for a person to develop this
disease?
ANS: Some of the genes implicated in heart disease are listed
in Table 19.2. Environmental factors might include diet,
amount of exercise, and whether or not the person
smokes.
19.2 A wheat variety with red kernels (genotype A′A′ B′B′)
was crossed with a variety with white kernels (genotype
AA BB). The F
1
were intercrossed to produce an F
2
. If
each primed allele increases the amount of pigment in
the kernel by an equal amount, what phenotypes will be
expected in the F
2
? Assuming that the A and B loci assort
independently, what will the phenotypic frequencies be?
ANS: 1/16 red; 4/16 1/4 dark pink; 6/16 pink; 4/16 1/4
light pink; 1/16 white.
19.3 For alcoholism, the concordance rate for monozygotic
twins is 55 percent, whereas for dizygotic twins, it is
28 percent. Do these data suggest that alcoholism has a
genetic basis?
ANS: The concordance for monozygotic twins is almost twice
as great as that for dizygotic twins. Monozygotic twins
share twice as many genes as dizygotic twins. The data
strongly suggest that alcoholism has a genetic basis.
19.4 The height of the seed head in wheat at maturity is deter-
mined by several genes. In one variety, the head is just
9 inches above the ground; in another, it is 33 inches
above the ground. Plants from the 9-inch variety were
crossed to plants from the 33-inch variety. Among the F
1
,
the seed head was 21 inches above the ground. After self-
fertilization, the F
1
plants produced an F
2
population in
which 9-inch and 33-inch plants each appeared with a
frequency of 1/256. (a) How many genes are involved in
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 79 8/14/2015 6:43:03 PM

80-WC Answers to All Questions and Problems
the determination of seed head height in these strains of
wheat? (b) How much does each allele of these genes
contribute to seed head height? (c) If a 21-inch F
1
plant
were crossed to a 9-inch plant, how often would you
expect 18-inch wheat to occur in the progeny?
ANS: (a) 4; (b) 3 inches; (c) frequency of 1/4.
19.5 Assume that size in rabbits is determined by genes with
equal and additive effects. From a total of 2012 F
2
prog-
eny from crosses between true-breeding large and small
varieties, eight rabbits were as small as the small variety
and eight were as large as the large variety. How many
size-determining genes were segregating in these
crosses?
ANS: Because 8/2012 is approximately 1/256 (1/4)
4
, it appears
that four size-determining genes were segregating in the
crosses.
19.6 A sample of 20 plants from a population was measured in
inches as follows: 18, 21, 20, 23, 20, 21, 20, 22, 19, 20, 17,
21, 20, 22, 20, 21, 20, 22, 19, and 23. Calculate (a) the
mean, (b) the variance, and (c) the standard deviation.
ANS: (a) The mean is 20.45 inches. (b) The variance is 2.37
inches
2
. (c) The standard deviation is 1.54 inches.
19.7 Quantitative geneticists use the variance as a measure of
scatter in a sample of data; they calculate this statistic by
averaging the squared deviations between each measure-
ment and the sample mean. Why don’t they simply mea-
sure the scatter by computing the average of the
deviations without bothering to square them?
ANS: Because S(X
i
− mean) 0.
19.8 Two inbred strains of corn were crossed to produce an
F
1
, which was then intercrossed to produce an F
2
. Data
on ear length from a sample of F
1
and F
2
individuals gave
phenotypic variances of 15.2 cm
2
and 27.6 cm
2
, respec-
tively. Why was the phenotypic variance greater for the
F
2
than for the F
1
?
ANS: For the F
1
, V
g
0 because they are all genetically identi-
cal and heterozygous; for the F
2
, V
g
> 0 because genetic
differences result from the segregation and independent
assortment of genes. Thus, in the F
2
, the phenotypic
variance has a pronounced genetic component.
19.9 A study of quantitative variation for abdominal bristle
number in female Drosophila yielded estimates of V
T
6.08, V
g
3.17, and V
e
2.91. What was the broad-sense
heritability?
ANS: 3.17/6.08 0.52
19.10 A researcher has been studying kernel number on ears of
corn. In one highly inbred strain, the variance for kernel
number is 426. Within this strain, what is the broad-
sense heritability for kernel number?
ANS: The broad-sense heritability within a highly inbred
strain is expected to be zero because there is no genetic
variability.
19.11 Measurements on ear length were obtained from three
populations of corn—two inbred varieties and a ran-
domly pollinated population derived from a cross
between the two inbred strains. The phenotypic vari-
ances were 9.2 cm
2
and 9.6 cm
2
for the two inbred variet-
ies and 26.4 cm
2
for the randomly pollinated population.
Estimate the broad-sense heritability of ear length for
these populations.
ANS: V
e
is estimated by the average of the variances of the
inbreds: 9.4 cm
2
. V
g
is estimated by the difference between
the variances of the randomly pollinated population and
the inbreds: (26.4 9.4) 17.0 cm
2
. The broad-sense
heritability is H
2
V
g
/V
T
17.0/26.4 0.64.
19.12 Figure 19.4 summarizes data on maturation time in pop-
ulations of wheat. Do these data provide any insight as to
whether or not this trait is inuenced by dominance?
Explain.
ANS: Because the F
1
plants have maturation times midway
between those of the parental strains, there seems to be
little or no dominance for this trait.
19.13 A quantitative geneticist claims that the narrow-sense
heritability for body mass in human beings is 0.7, while
the broad-sense heritability is only 0.3. Why must there
be an error?
ANS: Broad-sense heritability must be greater than narrow-
sense heritability because H
2
V
g
/V
T
> V
a
/V
T
h
2
.
19.14 The mean value of a trait is 100 units, and the narrow-
sense heritability is 0.4. A male and a female measuring
124 and 126 units, respectively, mate and produce a large
number of offspring, which are reared in an average
environment. What is the expected value of the trait
among these offspring?
ANS: (125 100)(0.4) 100 110 units.
19.15 The narrow-sense heritability for abdominal bristle
number in a population of Drosophila is 0.3. The mean
bristle number is 12. A male with 10 bristles is mated to
a female with 20 bristles, and a large number of progeny
are scored for bristle number. What is the expected num-
ber of bristles among these progeny?
ANS: (15 12)(0.3) 12 12.9 bristles.
19.16 A breeder is trying to decrease the maturation time in a
population of sunowers. In this population, the mean
time to owering is 100 days. Plants with a mean ower-
ing time of only 90 days were used to produce the next
generation. If the narrow-sense heritability for owering
time is 0.2, what will the average time to owering be in
the next generation?
ANS: (90 100)(0.2) 100 98 days.
19.17 A sh breeder wishes to increase the rate of growth in a
stock by selecting for increased length at 6 weeks after
hatching. The mean length of 6-week-old ngerlings is
currently 10 cm. Adult sh that had a mean length of
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 80 8/14/2015 6:43:03 PM

Answers to All Questions and Problems WC-81
15cm at 6 weeks of age were used to produce a new gen-
eration of ngerlings. Among these, the mean length was
12.5 cm. Estimate the narrow-sense heritability of n-
gerling length at 6 weeks of age and advise the breeder
about the feasibility of the plan to increase growth rate.
ANS: h
2
R/S (12.5 10)/(15 10) 0.5; selection for
increased growth rate should be effective.
19.18 Leo’s IQ is 86 and Julie’s IQ is 110. The mean IQ in the
population is 100. Assume that the narrow-sense herita-
bility for IQ is 0.4. What is the expected IQ of Leo and
Julie’s rst child?
ANS: (98 100)(0.4) 100 99.2.
19.19 One way to estimate a maximum value for the narrow-
sense heritability is to calculate the correlation between
half-siblings that have been reared apart and divide it by
the fraction of genes that half-siblings share by virtue of
common ancestry. A study of human half-siblings found
that the correlation coefcient for height was 0.14. From
this result, what is the maximum value of the narrow-
sense heritability for height in this population?
ANS: Half-siblings share 25 percent of their genes. The maxi-
mum value for h
2
is therefore 0.14/0.25 0.56.
19.20 A selection differential of 40 mg per generation was used
in an experiment to select for increased pupa weight in
Tribolium. The narrow-sense heritability for pupa weight
was estimated to be 0.3. If the mean pupa weight was
initially 2000 mg and selection was practiced for 10 gen-
erations, what was the mean pupa weight expected to
become?
ANS: The response to selection in one generation is R h
2
S
(0.3)(40 mg) 12 mg. The cumulative effect over 10 gen-
erations is therefore 10 12 mg 120 mg. Thus, the
mean pupa weight should become 2120 mg.
19.21 On the basis of the observed correlations for personality
traits shown in Table 19.5, what can you say about the
value of the environmentality (C
2
in Table 19.3)?
ANS: The correlations for MZT are not much different from
those for MZA. Evidently, for these personality traits,
the environmentality (C
2
in Table 19.3) is negligible.
19.22 Correlations between relatives provide estimates of the
broad and narrow-sense heritabilities on the assumption
that the genetic and environmental factors inuencing
quantitative traits are independent of each other and that
they do not interact in some peculiar way. In Chapter 18,
we considered epigenetic modications of chromatin
that regulate genes and noted the possibility that some of
these modications might be induced by environmental
factors. How could epigenetic inuences on complex
traits be incorporated into the basic theory of quantita-
tive genetics?
ANS: We might represent the value of a quantitative trait, T, as
m + g + e + eg, where m is the mean of the population, g is
the deviation due to genetic factors, e is the deviation due
to environmental factors, and eg is the deviation due to
epigenetic factors arising from the interaction of genetic
and environmental factors.
Chapter 20
20.1 The following data for the M–N blood types were
obtained from native villages in Central and North
America:
Group Sample Size M MN N
Central American 86 53 29 4
North American 278 78 61 139
Calculate the frequencies of the L
M
and L
N
alleles for the
two groups.
ANS: Frequency of L
M
in Central American population: p (2
53 29)/(2 86) 0.78; q 0.22. Frequency of L
M
in
North American population: p (2 78 61)/(2 278)
0.39; q 0.61.
20.2 The frequency of an allele in a large randomly mating
population is 0.2. What is the frequency of heterozygous
carriers?
ANS: 2pq 2(0.2)(0.8) 0.32.
20.3 The incidence of recessive albinism is 0.0004 in a human
population. If mating for this trait is random in the pop-
ulation, what is the frequency of the recessive allele?
ANS: q
2
0.0004; q 0.02.
20.4 In a sample from an African population, the frequencies
of the LM and LN alleles were 0.78 and 0.22, respec-
tively. If the population mates randomly with respect to
the M–N blood types, what are the expected frequencies
of the M, MN, and N phenotypes?
ANS:
Phenotype Hardy–Weinberg Frequency
M
(0.78)
2
0.61
MN
2(0.78)(0.22) 0.34
N
(0.22)
2
0.05
20.5 Human beings carrying the dominant allele T can taste
the substance phenylthiocarbamide (PTC). In a popula-
tion in which the frequency of this allele is 0.4, what is
the probability that a particular taster is homozygous?
ANS: Frequency of tasters (genotypes TT and Tt): (0.4)
2
2
(0.4)(0.6) 0.64. Frequency of TT tasters among all
tasters: (0.4)
2
/(0.64) 0.25.
20.6 A gene has three alleles, A
1
, A
2
, and A
3
, with frequencies
0.6, 0.3, and 0.1, respectively. If mating is random, pre-
dict the combined frequency of all the heterozygotes in
the population.
ANS: Frequency of heterozygotes combined 2[(0.6)(0.3)
(0.3)(0.1) ((0.6)(0.1)] 0.54.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 81 8/14/2015 6:43:03 PM

82-WC Answers to All Questions and Problems
20.7 Hemophilia is caused by an X-linked recessive allele. In a
particular population, the frequency of males with hemo-
philia is 1/4000. What is the expected frequency of
females with hemophilia?
ANS: (0.00025)
2
6.25 10
8
.
20.8 In Drosophila, the ruby eye phenotype is caused by a
recessive, X-linked mutant allele. The wild-type eye
color is red. A laboratory population of Drosophila is
started with 25 percent ruby-eyed females, 25 percent
homozygous red-eyed females, 5 percent ruby-eyed
males, and 45 percent red-eyed males. (a) If this popula-
tion mates randomly for one generation, what is the
expected frequency of ruby-eyed males and females? (b)
What is the frequency of the recessive allele in each of
the sexes?
ANS: (a) Half the males will be ruby-eyed and 5 percent (0.50
0.10 100 percent) of the females will be ruby-eyed.
(b) Among males, the frequency of the recessive allele
will be 0.5, which was its frequency among females in the
previous generation; among females, the frequency of
the recessive allele will be (0.5 0.1)/2 0.3, which is
the average of the frequencies of this allele in males and
females in the previous generation.
20.9 A trait determined by an X-linked dominant allele shows
100 percent penetrance and is expressed in 36 percent of
the females in a population. Assuming that the popula-
tion is in Hardy–Weinberg equilibrium, what proportion
of the males in this population express the trait?
ANS: In females, the frequency of the dominant phenotype is
0.36. The frequency of the recessive phenotype is 0.64 q
2
;
thus, q 0.8 and p 0.2. The frequency of the dominant
phenotype in males is therefore p 0.2.
20.10 A phenotypically normal couple has had one normal
child and a child with cystic brosis, an autosomal reces-
sive disease. The incidence of cystic brosis in the popu-
lation from which this couple came is 1/500. If their
normal child eventually marries a phenotypically normal
person from the same population, what is the risk that
the newlyweds will produce a child with cystic brosis?
ANS: The probability that the unaffected child of the couple is
a carrier of the mutant allele for cystic brosis is 2/3. The
probability that the mate of this individual is a carrier can
be determined by using the population incidence of the
disease. The mutant allele frequency is the square root of
the incidence—0.045—and the frequency of heterozy-
gotes under the assumption of random mating is 2
0.045 (1 0.045) 0.086. If both individuals are car-
riers, the chance that they will have an affected child is
1/4. Putting all this analysis together, the risk for the
child to have cystic brosis is therefore 2/3 0.086
1/4 0.014, which is seven times the incidence in the
population at large.
20.11 What frequencies of alleles A and a in a randomly mating
population maximize the frequency of heterozygotes?
ANS: Frequency of heterozygotes H 2pq 2p(1 p).
Using calculus, take the derivative of H and set the result
to zero to solve for the value of p that maximizes H: dH/
dp 2 4p 0 implies that p 2/4 0.5.
20.12 In an isolated population, the frequencies of the I
A
, I
B
,
and i alleles of the A–B–O blood type gene are, respec-
tively, 0.15, 0.25, and 0.60. If the genotypes of the A–B–O
blood type gene are in Hardy–Weinberg proportions,
what fraction of the people who have type A blood in this
population is expected to be homozygous for the I
A
allele?
ANS: In a Hardy–Weinberg population, the frequency of I
A
I
A
homozygotes is (0.15)
2
0.0335 and the frequency of I
A
i
heterozygotes is 2 0.15 0.60 0.18. The sum of
these frequencies—0.2025—is the frequency of individ-
uals with type A blood. Thus, the frequency of I
A
I
A
homozygotes among all individuals with type A blood is
0.0225/0.2025 0.1111.
20.13 In a survey of moths collected from a natural population,
a researcher found 51 dark specimens and 49 light speci-
mens. The dark moths carry a dominant allele, and the
light moths are homozygous for a recessive allele. If the
population is in Hardy–Weinberg equilibrium, what is
the estimated frequency of the recessive allele in the
population? How many of the dark moths in the sample
are likely to be homozygous for the dominant allele?
ANS: Under the assumption that the population is in Hardy–
Weinberg equilibrium, the frequency of the allele for
light coloration is the square root of the frequency of
recessive homozygotes. Thus, q √0.49 0.7, and the
frequency of the allele for dark color is 1 q p 0.3.
From p
2
0.09, we estimate that 0.09 100 9 of
the dark moths in the sample are homozygous for the
dominant allele.
20.14 A population of Hawaiian Drosophila is segregating two
alleles, P
1
and P
2
, of the phosphoglucose isomerase (PGI)
gene. In a sample of 100 ies from this population, 30
were P
1
P
1
homozygotes, 60 were P
1
P
2
heterozygotes, and
10 were P
2
P
2
homozygotes. (a) What are the frequencies
of the P
1
and P
2
alleles in this sample? (b) Perform
a chi-square test to determine if the genotypes in the
sample are in Hardy–Weinberg proportions. (c) Assum-
ing that the sample is representative of the population,
how many generations of random mating would be
required to establish Hardy–Weinberg proportions in
the population?
ANS: (a) P
1
0.6 and P
2
0.4. (b) Predict H–W proportions
by multiplying the expected genotype frequencies by the
sample size and compare these values with the observed
genotype frequencies using a chi-square test:
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 82 8/14/2015 6:43:03 PM
Answers to All Questions and Problems WC-83
Genotype H–W Frequency Predicted Number
P
1
P
1
0.6
2
0.36 0.36 100 36
P
1
P
2
2(0.6)(0.4) 0.48 0.28 100 48
P
2
P
2
0.4
2
0.16 0.16 100 16
c
2
(30 36)
2
/36 (60 48)
2
/48 (10 16)
2
/16 6.25,
df 1; 6.25 is greater than 3.841; therefore, the observed
genotypes are not in agreement with H–W.
20.15 In a large population that reproduces by random mating,
the frequencies of the genotypes GG, Gg, and gg are 0.04,
0.32, and 0.64, respectively. Assume that a change in the
climate induces the population to reproduce exclusively
by self-fertilization. Predict the frequencies of the geno-
types in this population after many generations of
self-fertilization.
ANS: Ultimate frequency of GG is 0.2; ultimate frequency of
gg is 0.8.
20.16 The frequencies of the alleles A and a are 0.6 and 0.4,
respectively, in a particular plant population. After many
generations of random mating, the population goes
through one cycle of self-fertilization. What is the
expected frequency of heterozygotes in the progeny of
the self-fertilized plants?
ANS: 2pq(1 F) 2(0.6)(0.4)(1 0.5) 0.24.
20.17 Each of two isolated populations is in Hardy–Weinberg
equilibrium with the genotype frequencies shown below:
Genotype: AA Aa Aa
Frequency in
Population 1:
0.04 0.32 0.64
Frequency in
Population 2:
0.64 0.32 0.04
(a) If the populations are equal in size and they merge to
form a single large population, predict the allele and
genotype frequencies in the large population immedi-
ately after merger.
(b) If the merged population reproduces by random mat-
ing, predict the genotype frequencies in the next
generation.
(c) If the merged population continues to reproduce by
random mating, will these genotype frequencies remain
constant?
ANS: (a) Frequency of A in merged population is 0.5 and that
of a is also 0.5; (b) 0.25 (AA), 0.50 (Aa), and 0.25 (aa);
(c) frequencies in (b) will persist.
20.18 A population consists of 25 percent tall individuals
(genotype TT), 25 percent short individuals (genotype
tt), and 50 percent individuals of intermediate height
(genotype Tt). Predict the ultimate phenotypic and
genotypic composition of the population if, generation
after generation, mating is strictly assortative (i.e., tall
individuals mate with tall individuals, short individuals
mate with short individuals, and intermediate individuals
mate with intermediate individuals).
ANS: Ultimately, all members of the population will either be
TT or tt, each 50% of the total.
20.19 In controlled experiments with different genotypes of
an insect, a researcher has measured the probability of
survival from fertilized eggs to mature, breeding
adults. The survival probabilities of the three geno-
types tested are 0.92 (for GG), 0.90 (for Gg), and 0.56
(for gg). If all breeding adults are equally fertile, what
are the relative tnesses of the three genotypes? What
are the selection coefcients for the two least t
genotypes?
ANS: The relative tnesses can be obtained by dividing each of
the survival probabilities by the largest probability (0.92).
Thus, the relative tnesses are 1 for GG, 0.98 1 0.02
for Gg, and 0.61 1 0.39 for gg. The selection coef-
cients are s
1
0.02 for Gg and s
2
0.39 for gg.
20.20 In a large randomly mating population, 0.84 of the indi-
viduals express the phenotype of the dominant allele A
and 0.16 express the phenotype of the recessive allele a.
(a) What is the frequency of the dominant allele? (b) If
the aa homozygotes are 5 percent less t than the other
two genotypes, what will the frequency of A be in the
next generation?
ANS: Frequency of a is q 0.4; (a) thus p 1 q 0.6;
(b) use the following scheme:
Genotype AA Aa Aa
Hardy–Weinberg frequency 0.36 0.48 0.16
Relative tness 1 1 0.95
Relative contribution to
next generation
0.36 0.48 0.152
Proportional contribution
to next generation
0.363 0.484 0.153
Thus, the frequency of the A allele in the next generation
will be (0.363 0.484/2) 0.605.
20.21 Because individuals with cystic brosis die before they
can reproduce, the coefcient of selection against them
is s 1. Assume that heterozygous carriers of the
recessive mutant allele responsible for this disease are as
t as wild-type homozygotes and that the population
frequency of the mutant allele is 0.02. (a) Predict the
incidence of cystic brosis in the population after one
generation of selection. (b) Explain why the incidence of
cystic brosis hardly changes even with s 1.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 83 8/14/2015 6:43:03 PM

84-WC Answers to All Questions and Problems
ANS: (a) Use the following scheme:
Genotype CC Cc cc
Hardy–Weinberg (0.98)
2
2(0.98)(0.02) (0.02)
2
frequency 0.9604 0.0392 0.0004
Relative tness 1 1 0
Relative (0.9604) 1 (0.0392) 1 0
contribution
to next generation
Proportional 0.9604/0.9996 0.0392/0.9996 0
contribution 0.9608 0.0392
The new frequency of the allele for cystic brosis is (0.5)
(0.0392) 0.0196; thus, the incidence of the disease will
be (0.0196)
2
0.00038, which is very slightly less than
the incidence in the previous generation. (b) The inci-
dence of cystic brosis does not change much because
selection can only act against the recessive allele when it
is in homozygotes, which are rare in the population.
20.22 For each set of relative tnesses for the genotypes AA,
Aa, and aa, explain how selection is operating. Assume
that 0 < t < s < 1.
AA Aa aa
Case 1 1 1 1 - s
Case 2 1 - s 1 - s 1
Case 3 1 1 - t 1 - s
Case 4 1 - s 1 1 - t
ANS: Case 1: selection is operating against a deleterious
recessive allele. Case 2: selection is operating against a
deleterious dominant allele. Case 3: selection is operat-
ing against a deleterious allele that has some expression
in heterozygotes, that is, it is partially dominant. Case 4:
selection is operating against both alleles in homozygous
condition; this is a case of balancing selection.
20.23 The frequency of newborn infants homozygous for a
recessive lethal allele is about 1 in 25,000. What is the
expected frequency of carriers of this allele in the
population?
ANS: q
2
4 10
5
; thus q 6.3 10
3
and 2pq 0.0126.
20.24 A population of size 50 reproduces in such a way that
the population size remains constant. If mating is ran-
dom, how rapidly will genetic variability, as measured by
the frequency of heterozygotes, be lost from this
population?
ANS: The frequency of heterozygotes will decrease by a
1/(2N) 1/100 0.01 per generation.
20.25 A population is segregating three alleles, A
1
, A
2
, and A
3
,
with frequencies 0.2, 0.5, and 0.3, respectively. If these
alleles are selectively neutral, what is the probability that
A
2
will ultimately be xed by genetic drift? What is the
probability that A
3
will ultimately be lost by genetic drift?
ANS: Probability of ultimate xation of A
2
is 0.5; probability of
ultimate loss of A
3
is 1 0.3 0.7.
20.26 A small island population of mice consists of roughly
equal numbers of males and females. The Y chromo-
some in one-fourth of the males is twice as long as the
Y chromosome in the other males because of an expan-
sion of heterochromatin. If mice with the large Y chro-
mosome have the same tness as mice with the small
Y chromosome, what is the probability that the large
Y chromosome will ultimately be xed in the mouse
population?
ANS: 0.25.
20.27 In some regions of west Africa, the frequency of the
HBB
S
allele is 0.2. If this frequency is the result of a
dynamic equilibrium due to the superior tness of
HBB
S
HBB
A
heterozygotes, and if HBB
S
HBB
S
homozy-
gotes are essentially lethal, what is the intensity of selec-
tion against the HBB
A
HBB
A
homozygotes?
ANS: p 0.2; at equilibrium, p t/(s t). Because s 1, we
can solve for t; t 0.25.
20.28 Mice with the genotype Hh are twice as t as either of
the homozygotes HH and hh. With random mating, what
is the expected frequency of the h allele when the mouse
population reaches a dynamic equilibrium because of
balancing selection?
ANS: The relative tnesses of the genotypes HH, Hh, and hh
are 0.5, 1, and 0.5, respectively. At equilibrium, the fre-
quency of h will be s/(t s) (0.5)/(0.5 0.5) 0.5.
20.29 A completely recessive allele g is lethal in homozygous
condition. If the dominant allele G mutates to g at a rate
of 10
6
per generation, what is the expected frequency of
the lethal allele when the population reaches mutation–
selection equilibrium?
ANS: At mutation–selection equilibrium
qus
== =
//
10 10
.001.
−6
20.30 Individuals with the genotype bb are 20 percent less t
than individuals with the genotypes BB or Bb. If B
mutates to b at a rate of 10
6
per generation, what is the
expected frequency of the allele b when the population
reaches mutation–selection equilibrium?
ANS:
qus== = 2.2 ×//(0.2)10 10
−−63
.
Chapter 21
21.1 Which of the following pairs of DNA sequences could
qualify as the terminal repeats of a bacterial IS element.
Explain.
(a) 5′-GAATCCGCA-3′ and 5′-ACGCCTAAG-3′
(b) 5′-GAATCCGCA-3′ and 5′-CTTAGGCGT-3′
(c)5′-GAATCCGCA-3′ and 5′-GAATCCGCA-3′
(d) 5′-GAATCCGCA-3′ and 5′-TGCGGATTC-3′.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 84 8/14/2015 6:43:05 PM

Answers to All Questions and Problems WC-85
ANS: The pair in (d) are inverted repeats and could therefore
qualify.
21.2 Which of the following pairs of DNA sequences could
qualify as target site duplications at the point of an IS50
insertion? Explain.
(a) 5′-AATTCGCGT-3′ and 5′-AATTCGCGT-3′
(b) 5′-AATTCGCGT-3′ and 5′-TGCGCTTAA-3′
(c) 5′-AATTCGCGT-3′ and 5′-TTAAGCGCA-3′
(d) 5′-AATTCGCGT-3′ and 5′-ACGCGAATT-3′.
ANS: The pair in (a) are direct repeats and could therefore
qualify.
21.3 One strain of E. coli is resistant to the antibiotic strepto-
mycin, and another strain is resistant to the antibiotic
ampicillin. The two strains were cultured together and
then plated on selective medium containing streptomy-
cin and ampicillin. Several colonies appeared, indicating
that cells had acquired resistance to both antibiotics.
Suggest a mechanism to explain the acquisition of dou-
ble resistance.
ANS: Resistance for the second antibiotic was acquired by con-
jugative gene transfer between the two types of cells.
21.4 What distinguishes IS and Tn3 elements in bacteria?
ANS: Tn3 elements carry a gene that is not essential for
transposition.
21.5 The circular order of genes on the E. coli chromosome is
*A B C D E F G H*, with the * indicating that the ends of
the chromosome are attached to each other. Two copies
of an IS element are located in this chromosome: one
between genes C and D, and the other between genes D
and E. A single copy of this element is also present in the
F plasmid. Two Hfr strains were obtained by selecting
for integration of the F plasmid into the chromosome.
During conjugation, one strain transfers the chromo-
somal genes in the order D E F G H A B C, whereas the
other transfers them in the order D C B A H G F E.
Explain the origin of these two Hfr strains. Why do they
transfer genes in different orders? Does the order of
transfer reveal anything about the orientation of the IS
elements in the E. coli chromosome?
ANS: In the rst strain, the F factor integrated into the chro-
mosome by recombination with the IS element between
genes C and D. In the second strain, it integrated by
recombination with the IS element between genes D and
E. The two strains transfer their genes in different orders
because the two chromosomal IS elements are in oppo-
site orientation.
21.6 The composite transposon Tn5 consists of two IS50 ele-
ments, one on either side of a group of three genes for
antibiotic resistance. The entire unit IS50L kan
r
ble
r
str
r
IS50R can transpose to a new location in the E. coli chro-
mosome. However, of the two IS50 elements in this
transposon, only IS50R produces the catalytically active
transposase. Would you expect IS50R to be able to be
excised from the Tn5 composite transposon and insert
elsewhere in the chromosome? Would you expect IS50L
to be able to do this?
ANS: Both IS50 elements should be able to excise from the
transposon and insert elsewhere in the chromosome,
because even though IS50L does not produce its own
transposase, IS50R provides a source of this enzyme.
21.7 By chance, an IS1 element has inserted near an IS2 ele-
ment in the E. coli chromosome. The gene between them,
sug
, confers the ability to metabolize certain sugars.
Will the unit IS1 sug
IS2 behave as a composite trans-
poson? Explain.
ANS: No. IS1 and IS2 are mobilized by different transposases.
21.8 A researcher has found a new Tn5 element with the
structure IS50L str
r
ble
r
kan
r
IS50L. What is the most
likely origin of this element?
ANS: IS50L inserted on each side of the cluster of antibiotic
resistance genes.
21.9 Would a Tn3 element with a frameshift mutation early
in the tnpA gene be able to form a cointegrate? Would a
Tn3 element with a frameshift mutation early in the
tnpR gene be able to form a cointegrate?
ANS: The tnpA mutation: no; the tnpR mutation: yes.
21.10 What enzymes are necessary for replicative transposition
of Tn3? What are their respective functions?
ANS: Two enzymes, transposase and resolvase, are needed for
replicative transposition. These enzymes are encoded by
genes of Tn3. Transposase catalyzes formation of a coin-
tegrate between donor and recipient plasmids. During
this process, Tn3 is replicated so that there is a copy of it
at each junction in the cointegrate. Resolvase catalyzes
the site-specic recombination between the two Tn3
elements and thereby resolves the cointegrate, generat-
ing two molecules each with a copy of the transposon.
Resolvase also represses the synthesis of both the trans-
posase and resolvase enzymes.
21.11 What is the medical signicance of bacterial transposons?
ANS: Many bacterial transposons carry genes for antibiotic
resistance, and it is relatively simple for these genes to
move from one DNA molecule to another. DNA mole-
cules that acquire resistance genes can be passed to other
cells in a bacterial population, both vertically (by descent)
and horizontally (by conjugative transfer). Over time,
continued exposure to an antibiotic will select for cells
that have acquired a gene for resistance to that antibiotic.
The antibiotic will therefore no longer be useful in com-
bating these bacteria.
21.12 Describe the structure of the Ac transposon in maize. In
what ways do the Ds transposons differ structurally and
functionally from the Ac transposon?
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 85 8/14/2015 6:43:05 PM
86-WC Answers to All Questions and Problems
ANS: The Ac element consists of 4563 nucleotide pairs
bounded by inverted repeats that are 11 nucleotide pairs
long. The Ac element is anked by direct repeats of eight
nucleotide pairs long; however, these repeats are created
at the time the element is inserted into a chromosome
(target site duplications) and are therefore not consid-
ered to be integral parts of the element itself. Ds ele-
ments possess the same terminal inverted repeats as Ac,
but their internal sequences vary. Some residue of the Ac
sequence may be present, or non-Ac sequences may be
present; sometimes, one Ds element is contained within
another Ds element.
21.13 In homozygous condition, a deletion mutation of the c
locus, c
n
, produces colorless (white) kernels in maize; the
dominant wild-type allele, C, causes the kernels to be
purple. A newly identied recessive mutation of the c
locus, c
m
, has the same phenotype as the deletion muta-
tion (white kernels), but when c
m
c
m
and c
n
c
n
plants are
crossed, they produce white kernels with purple stripes.
If it is known that the c
n
c
n
plants harbor Ac elements,
what is the most likely explanation for the c
m
mutation?
ANS: The c
m
mutation is due to a Ds or an Ac insertion.
21.14 In maize, the O2 gene, located on chromosome 7, con-
trols the texture of the endosperm, and the C gene,
located on chromosome 9, controls its color. The gene
on chromosome 7 has two alleles, a recessive, o2, which
causes the endosperm to be soft, and a dominant, O2,
which causes it to be hard. The gene on chromosome 9
also has two alleles, a recessive, c, which allows the endo-
sperm to be colored, and a dominant, C
I
, which inhibits
coloration. In one homozygous C
I
strain, a Ds element is
inserted on chromosome 9 between the C gene and the
centromere. This element can be activated by introduc-
ing an Ac element by appropriate crosses. Activation of
Ds causes the C
I
allele to be lost by chromosome break-
age. In C
I
/c/c kernels, such loss produces patches of col-
ored tissue in an otherwise colorless background.
A geneticist crosses a strain with the genotype o2/o2; C
I
Ds/C
I
Ds to a strain with the genotype O2/o2; c/c. The
latter strain also carries an Ac element somewhere in the
genome. Among the offspring, only those with hard
endosperm show patches of colored tissue. What does
this tell you about the location of the Ac element in the
O2/o2; c/c strain?
ANS: The Ac element must be tightly linked to the O2 allele.
21.15 In maize, the recessive allele bz (bronze) produces a lighter
color in the aleurone than does the dominant allele, Bz.
Ears on a homozygous bz/bz plant were fertilized by pol-
len from a homozygous Bz/Bz plant. The resulting cobs
contained kernels that were uniformly dark except for a
few on which light spots occurred. Suggest an
explanation.
ANS: The paternally inherited Bz allele was inactivated by a
transposable element insertion.
21.16 The X-linked singed locus is one of several in Drosophila
that controls the formation of bristles on the adult cuti-
cle. Males that are hemizygous for a mutant singed allele
have bent, twisted bristles that are often much reduced in
size. Several P element insertion mutations of the singed
locus have been characterized, and some have been
shown to revert to the wild-type allele by excision of the
inserted element. What conditions must be present to
allow such reversions to occur?
ANS: The P transposase to catalyze excision and the absence of
P-specic piRNAs that would repress excision.
21.17 Dysgenic hybrids in Drosophila have elevated mutation
rates as a result of P element transposition. How could
you take advantage of this situation to obtain P element
insertion mutations on the X chromosome?
ANS: Cross dysgenic (highly mutable) males carrying a wild-
type X chromosome to females homozygous for a bal-
ancer X chromosome; then cross the heterozygous F
1
daughters individually to their brothers and screen the
F
2
males that lack the balancer chromosome for mutant
phenotypes, including failure to survive (lethality). Muta-
tions identied in this screen are probably due to P ele-
ment insertions in X-linked genes.
21.18 If DNA from a P element insertion mutation of the Dro-
sophila white gene and DNA from a wild-type white gene
were puried, denatured, mixed with each other, rena-
tured, and then viewed with an electron microscope,
what would the hybrid DNA molecules look like?
ANS:
P element DNA
White gene DNA
(Should see a single-stranded DNA loop corresponding
to the insertion.)
21.19 When complete P elements are injected into embryos
from an M strain, they transpose into the chromosomes
of the germ line, and progeny reared from these embryos
can be used to establish new P strains. However, when
complete P elements are injected into embryos from
insects that lack these elements, such as mosquitoes, they
do not transpose into the chromosomes of the germ line.
What does this failure to insert in the chromosomes of
other insects indicate about the nature of P element
transposition?
ANS: Factors made by the y’s genome are required for trans-
position; other insects apparently lack the ability to pro-
vide these factors.
21.20 (a) What are retroviruslike elements? (b) Give examples
of retroviruslike elements in yeast and Drosophila.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 86 8/14/2015 6:43:05 PM

Answers to All Questions and Problems WC-87
(c) Describe how retroviruslike elements transpose.
(d) After a retroviruslike element has been inserted into
a chromosome, is it ever expected to be excised?
ANS: (a) Retroviruslike elements resemble integrated retrovi-
ruses in overall structure and behavior. (b) Examples
include the Ty1 element in yeast and the copia element in
Drosophila. (c) Retroviruslike elements transpose using
an RNA intermediate. The element DNA is transcribed
into single-stranded RNA, which is reverse-transcribed
into double-stranded DNA (cDNA). The double-
stranded cDNA is then inserted into a site in the genome.
(d) No. However, the LTRs could pair and recombine to
excise all but one LTR.
21.21 Sometimes, solitary copies of the LTR of Ty1 elements
are found in yeast chromosomes. How might these soli-
tary LTRs originate?
ANS: Through crossing over between the LTRs of a Ty1
element.
21.22 Would you ever expect the genes in a retrotransposon to
possess introns? Explain.
ANS: No. The intron sequences would be removed by RNA
processing prior to reverse transcription into DNA.
21.23 Suggest a method to determine whether the TART
retroposon is situated at the telomeres of each of the
chromosomes in the Drosophila genome.
ANS: In situ hybridization to polytene chromosomes using a
TART probe.
21.24 It has been proposed that the hobo transposable elements in
Drosophila mediate intrachromosomal recombination—
that is, two hobo elements on the same chromosome pair
and recombine with each other. What would such a
recombination event produce if the hobo elements were
oriented in the same direction on the chromosome?
What if they were oriented in opposite directions?
ANS: Same orientation: a deletion; opposite orientation: an
inversion.
21.25 What evidence suggests that some transposable elements
are not simply genetic parasites?
ANS: TART and HeT-A replenish the ends of Drosophila
chromosomes.
21.26 Approximately half of all spontaneous mutations in Dro-
sophila are caused by transposable element insertions. In
human beings, however, the accumulated evidence sug-
gests that the vast majority of spontaneous mutations are
not caused by transposon insertions. Propose a hypothe-
sis to explain this difference.
ANS: The transposition rate in humans may be very much less
than it is in Drosophila.
21.27 Z. Ivics, Z. Izsvák, and P. B. Hackett have “resurrected” a
nonmobile member of the Tc1/mariner family of trans-
posable elements isolated from the DNA of salmon.
These researchers altered 12 codons within the coding
sequence of the transposase gene of the salmon element
to restore the catalytic function of its transposase. The
altered element, called Sleeping Beauty, is being tested as
an agent for the genetic transformation of vertebrates
such as mice and zebra sh (and possibly humans).
Suppose that you have a bacterial plasmid containing the
gene for green uorescent protein (gfp) inserted between
the ends of a Sleeping Beauty element. How would you
go about obtaining mice or zebra sh that express the
gfp gene?
ANS: The Sleeping Beauty element could be used as a transfor-
mation vector in vertebrates much like the P element has
been used in Drosophila. The gfp gene could be inserted
between the ends of the Sleeping Beauty element and
injected into eggs or embryos along with an intact Sleep-
ing Beauty element capable of encoding the element’s
transposase. If the transposase that is produced in the
injected egg or embryo acts on the element that contains
the gfp gene, it might cause the latter to be inserted into
genomic DNA. Then, if the egg or embryo develops into
an adult, that adult can be bred to determine if a Sleeping
Beauty/gfp transgene is transmitted to the next genera-
tion. In this way, it would be possible to obtain strains of
mice or zebra sh that express the gfp gene.
21.28 The human genome contains about 5000 “processed
pseudogenes,” which are derived from the insertion of
DNA copies of mRNA molecules derived from many
different genes. Predict the structure of these pseudo-
genes. Would each type of processed pseudogene be
expected to found a new family of retrotransposons
within the human genome? Would the copy number of
each type of processed pseudogene be expected to
increase signicantly over evolutionary time, as the copy
number of the Alu family has? Explain your answers.
ANS: The processed pseudogenes will, in the best of cases,
contain sequences from the transcription start site to the
poly-A tail of the transcript (if there is one); however,
they will not contain the gene’s promoter or any of its
introns. Because these pseudogenes will not have a pro-
moter, they are not likely to found new retrotransposon
families. Without a promoter, they will not be tran-
scribed; hence, they will not produce RNA to be reverse
transcribed into DNA for insertion into other sites in the
genome. Most likely, the copy number of each of these
processed pseudogenes will not increase as the copy
number of the Alu element has. The Alu element con-
tains an “internal” promoter recognized by RNA poly-
merase III. Each insertion of the Alu element contains
this promoter and can therefore be transcribed into
RNA, which can subsequently be reverse transcribed
into DNA. The L1 element also contains an “internal”
promoter, but this promoter is recognized by RNA poly-
merase II. Most protein-coding genes contain an “exter-
nal” promoter—that is, one that is not transcribed—and
this promoter is recognized by RNA polymerase II.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 87 8/14/2015 6:43:05 PM

88-WC Answers to All Questions and Problems
Chapter 22
22.1 During oogenesis, what mechanisms enrich the cyto-
plasm of animal eggs with nutritive and determinative
materials?
ANS: Unequal division of the cytoplasm during the meiotic
divisions; transport of substances into the oocyte from
surrounding cells such as the nurse cells in Drosophila.
22.2 Predict the phenotype of a fruit y that develops from an
embryo in which the posterior pole cells had been
destroyed by a laser beam.
ANS: The y will be sterile because the posterior pole cells
form the germ line in adults of both sexes.
22.3 Outline the main steps in the genetic analysis of develop-
ment in a model organism such as Drosophila.
ANS: Collect mutations with diagnostic phenotypes; map the
mutations and test them for allelism with one another;
perform epistasis tests with mutations in different genes;
clone individual genes and analyze their function at the
molecular level.
22.4 Why is the early Drosophila embryo a syncytium?
ANS: Mitotic division is so rapid that there is not enough time
for membranes to form between cells.
22.5 In Drosophila, what larval tissues produce the external
organs of the adult?
ANS: Imaginal discs.
22.6 Like dorsal, bicoid is a strict maternal-effect gene in Dro-
sophila; that is, it has no zygotic expression. Recessive
mutations in bicoid (bcd) cause embryonic death by pre-
venting the formation of anterior structures. Predict the
phenotypes of (a) bcd/bcd animals produced by mating
heterozygous males and females; (b) bcd/bcd animals pro-
duced by mating bcd/bcd females with bcd/ males;
(c) bcd/ animals produced by mating bcd/bcd females
with bcd/ males; (d) bcd/bcd animals produced by mating
bcd/ females with bcd/bcd males; (e) bcd/ animals pro-
duced by mating bcd/ females with bcd/bcd males.
ANS: (a) Wild-type; (b) embryonic lethal; (c) embryonic lethal;
(d) wild-type; (e) wild-type.
22.7 Why do women, but not men, who are homozygous for
the mutant allele that causes phenylketonuria produce
children that are physically and mentally retarded?
ANS: In homozygous condition, the mutation that causes phe-
nylketonuria has a maternal effect. Women homozygous
for this mutation inuence the development of their
children in utero.
22.8 In Drosophila, recessive mutations in the dorsal–ventral
axis gene dorsal (dl) cause a dorsalized phenotype in
embryos produced by dl/dl mothers; that is, no ventral
structures develop. Predict the phenotype of embryos
produced by females homozygous for a recessive muta-
tion in the anterior–posterior axis gene nanos.
ANS: Some structures fail to develop in the posterior portion
of the embryo.
22.9 A researcher is planning to collect mutations in mater-
nal-effect genes that control the earliest events in
Drosophila development. What phenotype should the
researcher look for in this search for maternal-effect
mutations?
ANS: Female sterility. Females affected by these mutations will
lay abnormal eggs that will not develop into viable
embryos.
22.10 A researcher is planning to collect mutations in the gap
genes, which control the rst steps in the segmentation
of Drosophila embryos. What phenotype should the
researcher look for in this search for gap gene
mutations?
ANS: Screen for lethal mutations that prevent regions of the
embryo from developing normally.
22.11 How do the somatic cells that surround a developing
Drosophila egg in the ovary inuence the formation of
the dorsal–ventral axis in the embryo that will be pro-
duced after the egg is fertilized?
ANS: The somatic cells surrounding a developing Drosophila
egg in the ovary determine where the spätzle protein,
which is the ligand for the Toll receptor protein, will be
cleaved. This cleavage will eventually occur on the ven-
tral side of the developing embryo.
22.12 What events lead to a high concentration of hunchback
protein in the anterior of Drosophila embryos?
ANS: The hunchback mRNA is translated into protein only in
the anterior region of the developing embryo. This
RNA is supplied to the egg by the nurse cells and it is
also synthesized after fertilization by transcription of
the hunchback gene. This zygotic transcription is stimu-
lated by a transcription factor encoded by maternally
supplied bicoid mRNA, which is located in the anterior
of the egg. Thus, hunchback mRNA is concentrated in
the anterior of the embryo. In addition, the hunchback
mRNA that is located in the posterior of the embryo is
bound by nanos protein and then degraded. The nanos
protein is concentrated in the posterior of the embryo
because maternally supplied nanos mRNA is preferen-
tially localized there.
22.13 Diagram a pathway that shows the contributions of the
sevenless (sev) and bride of sevenless (boss) genes to the dif-
ferentiation of the R7 photoreceptor in the ommatidia of
Drosophila eyes. Where would eyeless (ey) t in this
pathway?
ANS: ey → boss → sev → R7 differentiation
22.14 The sev
B4
allele is temperature sensitive; at 22.7°C, ies
that are homozygous for it develop normal R7 photore-
ceptors, but at 24.3°C, they fail to develop these photo-
receptors. sos
2A
is a recessive, loss-of-function mutation
in the son of sevenless (sos) gene. Flies with the genotype
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 88 8/14/2015 6:43:05 PM

Answers to All Questions and Problems WC-89
sev
B4
/sev
B4
; sos
2A
/ fail to develop R7 photoreceptors
if they are raised at 22.7°C. Therefore, sos2A acts as
a dominant enhancer of the sev
B4
mutant phenotype
at this temperature. Based on this observation, where
is the protein product of the wild-type sos gene—
called SOS—likely to act in the pathway for R7
differentiation?
ANS: If the SEV protein is activated—either by the BOSS
ligand or by a gain-of-function mutation in the sev gene,
a faulty effector protein could stop it from inducing the
R7 cell to differentiate. The SOS protein is likely to be a
downstream effector in the pathway for R7 differentia-
tion because when it is depleted by mutating one copy of
the sos gene, ies that have a partially functional SEV
protein show a mutant phenotype—that is, transmission
of the developmental signal through SEV and its down-
stream effector proteins is weakened.
20.15 When the mouse Pax6 gene, which is homologous to the
Drosophila eyeless gene, is expressed in Drosophila, it pro-
duces extra compound eyes with ommatidia, just like
normal Drosophila eyes. If the Drosophila eyeless gene were
introduced into mice and expressed there, what effect
would you expect? Explain.
ANS: Because the Pax6 gave the same phenotype in ies as
overexpression of the eyeless gene, the genes must be
functionally homologous, as well as structurally homolo-
gous. Therefore, expect extra mouse eyes or eye pri-
morida when expressing eyeless in the mouse.
22.16 Would you expect to nd homologues of Drosophila’s
BX-C and ANT-C genes in animals with radial symme-
try such as sea urchins and starsh? How could you
address this question experimentally?
ANS: Maybe these organisms would not have homologues of
the BX-C and ANT-C genes because they do not have
segmented bodies with bilateral symmetry as Drosophila
does. To see if homologues to these genes are present,
use Drosophila BX-C and ANT-C DNA as probes to
hybridize with starsh or sea urchin genomic DNA on a
Southern blot. The hybridization would have to be done
under conditions that allow DNA that is not a perfect
match to form a duplex—that is, under conditions of low
stringency. Usually, hybridizations of this type are car-
ried out at lower temperatures than typical Southern
hybridizations. If the probes stick to the DNA on the
blot, there is evidence for homologues to the BX-C and
ANT-C genes in the genomic DNA. Follow-up experi-
ments might endeavor to clone this DNA and, ultimately,
to sequence it to determine just how close a match it is to
the Drosophila probe DNA.
22.17 How might you show that two mouse Hox genes are
expressed in different tissues and at different times dur-
ing development?
ANS: Northern blotting of RNA extracted from the tissues at
different times during development. Hybridize the blot
with gene-specic probes.
22.18 Distinguish between therapeutic and reproductive
cloning.
ANS: Therapeutic cloning involves the creation of an embryo
by implanting the nucleus of a somatic cell into an enu-
cleated egg and stimulating the egg to divide. Stem cells
are then taken from the embryo to differentiate into spe-
cic tissues in the individual from which the somatic cell
was taken. These tissues will be genetically identical to
the other tissues of the individual—thus, they are unlikely
to be rejected by the individual’s immune system. Repro-
ductive cloning involves the creation of an embryo by
implanting the nucleus of a somatic cell into an enucle-
ated egg and then allowing the egg to develop into an
entire individual.
22.19 What is the scientic signicance of reproductive
cloning?
ANS: Reproductive cloning of mammals such as sheep, mice,
and cats indicates that somatic cell nuclei have all the
genetic information to direct the development of a com-
plete, viable organism. It also shows that epigenetic
modications of chromatin, such as X chromosome inac-
tivation, can be reset.
22.20 The methylation of DNA, the acetylation of histones,
and the packaging of DNA into chromatin by certain
kinds of proteins are sometimes referred to as epigenetic
modications of the DNA. These modications portend
difculties for reproductive cloning. Do they also por-
tend difculties for therapeutic cloning and for the use of
stem cells to treat diseases or injuries that involve the loss
of specic cell types?
ANS: Methylation of DNA, acetylation of histones, and pack-
aging of DNA into chromatin by certain kinds of pro-
teins all portend difculties for therapeutic cloning as
well as for reproductive cloning. These epigenetic modi-
cations of somatic cell DNA would have to be “repro-
grammed” in the oocyte or they could affect how the
stem cells derived from the oocyte would develop.
22.21 Assume that an animal is capable of producing 100 mil-
lion different antibodies and that each antibody contains
a light chain of 220 amino acids long and a heavy chain
of 450 amino acids. How much genomic DNA would be
needed to accommodate the coding sequences of these
genes?
ANS: If each antibody consists of one kind of light chain and
one kind of heavy chain, and if light and heavy chains can
combine freely, the potential to produce 100 million dif-
ferent antibodies implies the existence of 10,000 light
chain genes and 10,000 heavy chain genes (10,000
10,000 100 million). If each light chain is 220 amino
acids long, each light chain gene must comprise 3 220
660 nucleotides because each amino acid is specied by a
triplet of nucleotides; similarly, each heavy chain gene
must comprise 3 450 1350 nucleotides. Therefore,
the genome must contain 10,000 660 6.6 million
nucleotides devoted to light chain production and
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 89 8/14/2015 6:43:05 PM

90-WC Answers to All Questions and Problems
10,000 1,350 13.5 million nucleotides devoted to
heavy chain production. Altogether, then, the genome
must contain 19.5 million nucleotides dedicated to
encoding the amino acids of the various antibody chains.
22.22 Each L
k
V
k
gene segment in the kappa light chain locus on
chromosome 2 consists of two coding exons, one for the
leader peptide and one for the variable portion of the
kappa light chain. Would you expect to nd a stop codon
at the end of the coding sequence in the second
(V
k
) exon?
ANS: No, because the V
k
coding sequence must be joined to
the coding sequence of the constant region to encode a
complete kappa light chain.
Chapter 23
23.1 Many cancers seem to involve environmental factors.
Why, then, is cancer called a genetic disease?
ANS: Cancer has been called a genetic disease because it results
from mutations of genes that regulate cell growth and
division. Nonhereditary forms of cancer result from
mutations in somatic cells. These mutations, however,
can be induced by environmental factors including
tobacco smoke, chemical pollutants, ionizing radiation,
and UV light. Hereditary forms of cancer also frequently
involve the occurrence of environmentally induced
somatic mutations.
23.2 Both embryonic cells and cancer cells divide quickly.
How can these two types of cells be distinguished from
each other?
ANS: Cancer cells do not display contact inhibition—they pile
up on top of each other—whereas embryonic cells spread
out in at sheets. Cancer cells are frequently aneuploid;
embryonic cells are euploid.
23.3 Most cancer cells are aneuploid. Suggest how aneuploidy
might contribute to deregulation of the cell cycle.
ANS: Aneuploidy might involve the loss of functional copies of
tumor suppressor genes, or it might involve the inappro-
priate duplication of proto-oncogenes. Loss of tumor sup-
pressor genes would remove natural brakes on cell division,
and duplication of proto-oncogenes would increase the
abundance of factors that promote cell division.
23.4 Would you ever expect to nd a tumor-inducing retrovi-
rus that carried a processed cellular tumor suppressor
gene in its genome?
ANS: No. A virus that carried a processed copy of a tumor sup-
pressor gene would not be expected to induce tumor for-
mation because the product of the tumor suppressor
gene would help to restrain cell growth and division.
23.5 How do we know that normal cellular oncogenes are not
simply integrated retroviral oncogenes that have acquired
the proper regulation?
ANS: They possess introns.
23.6 How might the absence of introns in a retroviral onco-
gene explain that gene’s overexpression in the tissues of
an infected animal?
ANS: The absence of introns might speed up the expression of
the gene’s protein product because there would be no
need for splicing. In addition, some introns contain
sequences called silencers that negatively regulate tran-
scription. Removal of these sequences might cause tran-
scription to occur when it otherwise would not.
23.7 When cellular oncogenes are isolated from different ani-
mals and compared, the amino acid sequences of the
polypeptides they encode are found to be very similar.
What does this suggest about the functions of these
polypeptides?
ANS: The products of these genes play important roles in cell
activities.
23.8 The majority of the c-ras oncogenes obtained from can-
cerous tissues have mutations in codon 12, 59, or 61 in
the coding sequence. Suggest an explanation.
ANS: Mutations in these codons cause amino acid changes that
activate the Ras protein.
23.9 When a mutant c-H-ras oncogene with a valine for gly-
cine substitution in codon 12 is transfected into cultured
NIH 3T3 cells, it transforms those cells into cancer cells.
When the same mutant oncogene is transfected into cul-
tured embryonic cells, it does not transform them. Why?
ANS: The cultured NIH 3T3 cells probably carry other muta-
tions that predispose them to become cancerous; trans-
fection of such cells with a mutant c-H-ras oncogene may
be the last step in the process of transforming the cells
into cancer cells. Cultured embryonic cells probably do
not carry the predisposing mutations needed for them to
become cancerous; thus, when they are transfected with
the mutant c-H-ras oncogene, they continue to divide
normally.
23.10 A mutation in the ras cellular oncogene can cause cancer
when it is in heterozygous condition, but a mutation in
the RB tumor suppressor gene can cause cancer only
when it is in homozygous condition. What does this dif-
ference between dominant and recessive mutations
imply about the roles that the ras and RB gene products
play in normal cellular activities?
ANS: Ras protein is an activator of cell division, whereas RB
protein is a suppressor of cell division.
23.11 Explain why individuals who develop nonhereditary reti-
noblastoma usually have tumors in only one eye, whereas
individuals with hereditary retinoblastoma usually
develop tumors in both eyes.
ANS: Retinoblastoma results from homozygosity for a loss-of-
function (recessive) allele. The sporadic occurrence of
retinoblastoma requires two mutations of this gene in
the same cell or cell lineage. Therefore, retinoblastoma
is rare among individuals who, at conception, are
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 90 8/14/2015 6:43:05 PM

Answers to All Questions and Problems WC-91
homozygous for the wild-type allele of the RB gene. For
such individuals, we would expect the frequency of
tumors in both eyes to be the square of the frequency of
tumors in one eye. Individuals who are heterozygous for
a mutant RB allele require only one somatic mutation to
occur for them to develop retinoblastoma. Because there
are millions of cells in each retina, there is a high prob-
ability that this somatic mutation will occur in at least
one cell in each eye, causing both eyes to develop tumors.
23.12 Approximately 5 percent of the individuals who inherit
an inactivated RB gene do not develop retinoblastoma.
Use this statistic to estimate the number of cell divisions
that form the retinal tissues of the eye. Assume that the
rate at which somatic mutations inactivate the RB gene is
one mutation per 10
6
cell divisions.
ANS: The probability that a carrier does not develop retino-
blastoma is 0.05, which is equal to the probability that
the wild-type RB allele is not mutationally inactivated
during the cell divisions that form the retinas of the eyes.
If the rate of mutational inactivation is u 10
6
per cell
division, and n is the number of cell divisions, then 0.05
(1 u)
n
. If we take logarithms of both sides, log(0.05)
nlog(1 u), which to a good approximation, is equal to
nlog (u). After substituting 10
6
for u and solving, we nd
that n 1.3 10
6
.
23.13 Inherited cancers like retinoblastoma show a dominant
pattern of inheritance. However, the underlying genetic
defect is a recessive loss-of-function mutation—often
the result of a deletion. How can the dominant pattern of
inheritance be reconciled with the recessive nature of the
mutation?
ANS: At the cellular level, loss-of-function mutations in the RB
gene are recessive; a cell that is heterozygous for such a
mutation divides normally. However, when a second
mutation occurs, that cell becomes cancerous. If the rst
RB mutation was inherited, there is a high probability
that the individual carrying this mutation will develop
retinoblastoma because a second mutation can occur any
time during the formation of the retinas in either eye.
Thus, the individual is predisposed to develop retino-
blastoma, and it is this predisposition that shows a domi-
nant pattern of inheritance.
23.14 The following pedigree shows the inheritance of familial
ovarian cancer caused by a mutation in the BRCA1 gene.
Should II-1 be tested for the presence of the predispos-
ing mutation? Discuss the advantages and disadvantages
of testing.
Ovarian cancer
Normal
I
II
III
ANS: II-1 should be tested for the BRCA1 mutation that appar-
ently was involved in the ovarian cancer that developed
in her mother and sister. If she is found to carry this
mutation, a prophylactic oophorectomy can be pre-
scribed to reduce the chance that she will develop
cancer. If she is found to be free of the mutation carried
by her mother and sister, then she is not more likely to
develop ovarian cancer than a woman in the general
population.
23.15 In what sense is pRB a negative regulator of E2F tran-
scription factors?
ANS: By binding to E2F transcription factors, pRB prevents
those transcription factors from activating their target
genes—which encode proteins involved in progression
of the cell cycle; pRB is therefore a negative regulator of
transcription factors that stimulate cell division.
23.16 A particular E2F transcription factor recognizes the
sequence TTTCGCGC in the promoter of its target
gene. A temperature-sensitive mutation in the gene
encoding this E2F transcription factor alters the ability
of its protein product to activate transcription; at 25°C,
the mutant protein activates transcription normally, but
at 35°C, it fails to activate transcription at all. However,
the ability of the protein to recognize its target DNA
sequence is not impaired at either temperature. Would
cells heterozygous for this temperature-sensitive muta-
tion be expected to divide normally at 25°C? at 35°C?
Would your answers change if the E2F protein functions
as a homodimer?
ANS: At 25°, cell division should be normal—the same as for
cells homozygous for a wild-type allele of the E2F gene.
At 35°, division would be expected to be impaired either
because the mutant E2F protein binds unproductively to
the sequence in its target gene or because a mutant E2F
polypeptide dimerizes with a wild-type E2F polypeptide
and abolishes the activation function of the wild-type
polypeptide.
23.17 During the cell cycle, the p16 protein is an inhibitor of
cyclin/CDK activity. Predict the phenotype of cells
homozygous for a loss-of-function mutation in the gene
that encodes p16. Would this gene be classied as a
proto-oncogene or as a tumor suppressor gene?
ANS: Cells homozygous for a loss-of-function mutation in the
p16 gene might be expected to divide in an uncontrolled
manner because the p16 protein would not be able to
inhibit cyclin-CDK activity during the cell cycle. The
p16 gene would therefore be classied as a tumor sup-
pressor gene.
23.18 The BCL-2 gene encodes a protein that represses the
pathway for programmed cell death. Predict the pheno-
type of cells heterozygous for a dominant activating
mutation in this gene. Would the BCL-2 gene be classi-
ed as a proto-oncogene or as a tumor suppressor gene?
ANS: Cells heterozygous for a dominant activating mutation
in the BCL-2 gene would be expected to be unable to
execute the programmed cell death pathway in response
to DNA damage induced by radiation treatment.
Such cells would continue to divide and accumulate
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 91 8/14/2015 6:43:06 PM

92-WC Answers to All Questions and Problems
mutations; ultimately they would have a good chance of
becoming cancerous. The BCL-2 gene would therefore
be classied as a proto-oncogene.
23.19 The protein product of the BAX gene negatively regu-
lates the protein product of the BCL-2 gene—that is,
BAX protein interferes with the function of the BCL-2
protein. Predict the phenotype of cells homozygous for a
loss-of-function mutation in the BAX gene. Would this
gene be classied as a proto-oncogene or as a tumor sup-
pressor gene?
ANS: Cells homozygous for a loss-of-function mutation in the
BAX gene would be unable to prevent repression of the
programmed cell death pathway by the BCL-2 gene
product. Consequently, these cells would be unable to
execute that pathway in response to DNA damage
induced by radiation treatment. Such cells would
continue to divide and accumulate mutations; ultimately,
they would have a good chance of becoming cancerous.
The BAX gene would therefore be classied as a tumor
suppressor gene.
23.20 Cancer cells frequently are homozygous for loss-of-
function mutations in the TP53 gene, and many of these
mutations map in the portion of TP53 that encodes the
DNA-binding domain of p53. Explain how these muta-
tions contribute to the cancerous phenotype of the cells.
ANS: Loss-of-function mutations in the DNA-binding
domain of p53 abolish the ability of that protein to acti-
vate transcription of target genes whose products are
involved in the restraint of cell division or in the promo-
tion of programmed cell death. Without restraint of cell
division or promotion of programmed cell death, cells
accumulate damage to their DNA and ultimately become
cancerous.
23.21 Suppose that a cell is heterozygous for a mutation that
caused p53 to bind tightly and constitutively to the DNA
of its target genes. How would this mutation affect the
cell cycle? Would such a cell be expected to be more or
less sensitive to the effects of ionizing radiation?
ANS: If a cell were heterozygous for a mutation that caused
p53 to bind tightly and constitutively to the DNA of its
target genes, its growth and division might be retarded,
or it might be induced to undergo apoptosis. Such a cell
would be expected to be more sensitive to the effects of
ionizing radiation because radiation increases the expres-
sion of p53, and in this case, the p53 would be predis-
posed to activate its target genes, causing the cell to
respond vigorously to the radiation treatment.
23.22 Mice homozygous for a knockout mutation of the TP53
gene are viable. Would they be expected to be more or
less sensitive to the killing effects of ionizing radiation?
ANS: Homozygous TP53 knockout mice might actually be less
sensitive to the killing effects of ionizing radiation
because p53 would be unable to mediate the apoptotic
response to the radiation treatment.
23.23 Would cancer-causing mutations of the APC gene be
expected to increase or decrease the ability of pAPC to
bind b-catenin?
ANS: They would probably decrease the ability of pAPC to
bind b-catenin.
23.24 Mice that are heterozygous for a knockout mutation in
the RB gene develop pituitary and thyroid tumors. Mice
that are homozygous for this mutation die during embry-
onic development. Mice that are homozygous for a
knockout mutation in the gene encoding the p130
homologue of RB and heterozygous for a knockout
mutation in the gene encoding the p107 homologue of
RB do not have a tendency to develop tumors. However,
homozygotes for knockout mutations in both of these
genes die during embryonic development. What do
these ndings suggest about the roles of the RB, p139,
and p107 genes in embryos and adults?
ANS: All three genes (RB, p130, and p107) are essential for
embryonic development, although by themselves, p130
and p107 are dispensable, possibly because their products
are functionally redundant. (Both gene products must be
inactivated before any deleterious effect is seen.) In
adults, only pRB appears to play a role in suppressing
tumor formation.
23.25 It has been demonstrated that individuals with diets poor
in ber and rich in fatty foods have an increased risk to
develop colorectal cancer. Fiber-poor, fat-rich diets may
irritate the epithelial lining of the large intestine. How
could such irritation contribute to the increased risk for
colorectal cancer?
ANS: The increased irritation to the intestinal epithelium
caused by a ber-poor, fat-rich diet would be expected to
increase the need for cell division in this tissue (to replace
the cells that were lost because of the irritation), with a
corresponding increase in the opportunity for the occur-
rence of cancer-causing mutations.
23.26 Messenger RNA from the KAI1 gene is strongly
expressed in normal prostate tissues but weakly expressed
in cell lines derived from metastatic prostate cancers.
What does this nding suggest about the role of the
KAI1 gene product in the etiology of prostate cancer?
ANS: The KAI1 gene is a prostate tumor suppressor gene.
Functional inactivation of this gene allows prostate
tumors to develop.
23.27 The p21 protein is strongly expressed in cells that have
been irradiated. Researchers have thought that this strong
expression is elicited by transcriptional activation of the
p21 gene by the p53 protein acting as a transcription factor.
Does this hypothesis t with the observation that p21
expression is induced by radiation treatment in mice homo-
zygous for a knockout mutation in the TP53 gene? Explain.
ANS: No. Apparently there is another pathway—one not medi-
ated by p53—that leads to the activation of the p21 gene.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 92 8/14/2015 6:43:06 PM
Answers to All Questions and Problems WC-93
Chapter 24
24.1 What was some of the evidence that led Charles Darwin
to argue that species change over time?
ANS: Among other things, Darwin observed species on islands
that were different from each other and from continental
species but were still similar enough to indicate that they
were related. He also observed variation within species,
especially within domesticated breeds, and saw how the
characteristics of an organism could be changed by selec-
tive breeding. His observations of fossilized organisms
indicated that some species have become extinct.
24.2 Darwin stressed that species evolve by natural selection.
What was the main gap in his theory?
ANS: Darwin did not understand the mechanism of inheri-
tance; he did not know of Mendel’s principles.
24.3 Using the data in Table 24.1, and assuming that mating is
random with respect to the blood type, predict the fre-
quencies of the three genotypes of the Duffy blood-type
locus in a South African and an English population.
ANS: The frequency of the a allele is 0.06 in the South African
population and 0.42 in the English population. The pre-
dicted genotype frequencies under the assumption of
random mating are as follows:
Genotype South Africa England
aa
(0.06)
2
0.004 (0.42)
2
0.18
ab
2(0.06)(0.94) 0.11 2(0.42)(0.58) 0.49
bb
(0.94)
2
0.88 (0.58)
2
0.33
24.4 Theodosius Dobzhansky and his collaborators studied
chromosomal polymorphisms in Drosophila pseudoobscura
and its sister species in the western United States. In one
study of polymorphisms in chromosome III of D. pseu-
doobscura sampled from populations at different locations
in the Yosemite region of the Sierra Nevada, Dobzhan-
sky (1948, Genetics 33: 158–176) recorded the following
frequencies of the Standard (ST) banding pattern:
Location Frequency ST
Elevation
(in feet)
Jacksonville 0.46 850
Lost Claim 0.41 3,000
Mather 0.32 4,600
Aspen 0.26 6,200
Porcupine 0.14 8,000
Tuolumne 0.11 8,600
Timberline 0.10 9,900
Lyell Base 0.10 10,500
What is interesting about these data?
ANS: The frequency of the ST banding pattern declines with
increasing altitude. Thus, the data indicate that this
chromosomal polymorphism exhibits an altitudinal
cline.
24.5 In a survey of electrophoretically detectable genetic vari-
ation in the alcohol dehydrogenase gene of Drosophila
melanogaster, a researcher found two allozymes, denoted
F (fast) and S (slow) in a population; 32 individuals were
homozygous for the F allele of the gene, 22 were homo-
zygous for the S allele, and 46 were heterozygous for the
F and S alleles. Are the observed frequencies of the three
genotypes consistent with the assumption that the popu-
lation is in Hardy–Weinberg equilibrium?
ANS: In the sample, the frequency of the F allele is (2 32
46)/(2 100) 0.55 and the frequency of the S allele is
1 0.55 0.45. The predicted and observed genotype
frequencies are as follows:
Genotype Observed Hardy–Weinberg Predicted
FF 32
100 (0.55)
2
30.25
FS 46
100 2(0.55)(0.45) 49.5
SS 22
100 (0.45)
2
20.25
To test for agreement between the observed and pre-
dicted values, we compute a chi-square statistic with
1 degree of freedom: c
2
S(obs. pred.)
2
/pred. 0.50,
which is not signicant at the 5 percent level. Thus, the
population appears to be in Hardy–Weinberg equilib-
rium for the alcohol dehydrogenase locus.
24.6 A researcher has been studying genetic variation in sh
populations by using PCR to amplify microsatellite
repeats at a particular site on a chromosome (see Chap-
ter 16). The diagram below shows the gel-fractionated
products of amplications with DNA samples from 10
different sh. How many distinct alleles of this microsat-
ellite locus are evident in the gel?
ANS: There are four alleles.
24.7 Within the coding region of a gene, where would you
most likely nd silent polymorphisms?
ANS: In the third position of some of the codons. Due to the
degeneracy of the genetic code, different codons can
specify the same amino acid. The degeneracy is most
pronounced in the third position of many codons, where
different nucleotides can be present without changing
the amino acid that is specied.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 93 8/14/2015 6:43:06 PM
94-WC Answers to All Questions and Problems
24.8 Why are the nucleotide sequences of introns more poly-
morphic than the nucleotide sequences of exons?
ANS: Introns do not encode amino acids; most exons do.
Many—perhaps most—of the nucleotides within an
intron can be changed without impairing the expression
of the gene or the integrity of its polypeptide product.
By contrast, many of the nucleotides within exons—
especially the rst and second positions of codons—are
functionally constrained by the amino acids specied.
24.9 DNA and protein molecules are “documents of evolu-
tionary history.” Why aren’t complex carbohydrate mol-
ecules such as starch, cellulose, and glycogen considered
“documents of evolutionary history”?
ANS: Complex carbohydrates are not “documents of evolu-
tionary history” because, although they are polymers,
they are typically made of one subunit incorporated rep-
etitiously into a chain. Such a polymer has little or no
“information content.” Thus, there is little or no oppor-
tunity to distinguish a complex carbohydrate obtained
from two different organisms. Moreover, complex carbo-
hydrates are not part of the genetic machinery; their for-
mation is ultimately specied by the action of enzymes,
which are gene products, but they themselves are not
genetic material or the products of the genetic material.
24.10 A geneticist analyzed the sequences of a gene cloned
from four different individuals. The four clones were
identical except for a few base pair differences, a deletion
(gap), and a transposable element (TE) insertion:
Sequences
1
TE
G
C
2
TE
A
T
T
A
G
C
C
G
3
C
G
4
A
T
Using this information, compute the minimum number
of mutations required to explain the derivation of the
four sequences (1, 2, 3, and 4) in the following phyloge-
netic trees:
12431234
Tree ATree BTree C
3421
Which of these trees provides the most parsimonious
explanation for the evolutionary history of the four DNA
sequences?
ANS: The tree on the right would require nine mutations.
These mutations are a deletion in the branch leading to
the common ancestor of sequences 1, 2, and 3; a TE
insertion in the branch leading to the common ancestor
of sequences 1 and 2; and seven base-pair changes: one
leading to sequence 1, another leading to sequence 2, two
leading to sequence 3 and three leading to sequence 4.
The tree in the middle would also require nine mutations.
In this tree, we regard the gap as ancestral—which means
that sequence 4 has acquired an “insertion” at the posi-
tion of the gap. The other mutations are a TE insertion
in the branch leading to the common ancestor of
sequences 1 and 2, and seven base-pair changes: one
leading to sequence 1, another to sequence 2, two lead-
ing to sequence 3, and three leading to sequence 4.
The tree on the right would also require nine mutations.
Here again we regard the gap as ancestral; evidently, an
insertion occurred at the position of the gap in the
branch leading to sequence 4. The TE insertion can also
be regarded as ancestral, with loss occurring in the
branch leading to the common ancestor of sequences
3 and 4. There are also seven base-pair changes in this
tree: one leading to sequence 1, another leading to
sequence 2, two leading to sequence 3 and three leading
to sequence 4. These interpretations of the data assume
that insertions and deletions (gaps) are reversible and
that there is no way of telling which way the sequence
evolved—that is, through insertion or deletion. How-
ever, if we know, for example, that the TE is a retrotrans-
poson incapable of excision, then we would need more
than nine mutations to explain the tree on the right. The
TE insertions must have occurred independently in the
branches leading to sequences 1 and 2, and there is no
need to postulate excision of the TE in the branch lead-
ing to the common ancestor of sequences 3 and 4. Thus,
on the assumption that the TE is not excisable, 10 muta-
tions are needed to explain the tree on the right. Given
this reservation, the left and middle trees provide the
most parsimonious explanations for the evolution of the
four sequences.
24.11 The heme group in hemoglobin is held in place by histi-
dines in the globin polypeptides. All vertebrate globins
possess these histidines. Explain this observation in terms
of the Neutral Theory of Molecular Evolution.
ANS: The histidines are rigorously conserved because they
perform an important function—anchoring the heme
group in hemoglobin. Because these amino acids are
strongly constrained by natural selection, they do not
evolve by mutation and random genetic drift.
24.12 During the early evolutionary history of the vertebrates,
a primordial globin gene was duplicated to form the
α- and b-globin genes. The rate of evolution of the poly-
peptides encoded by these duplicate genes has been
estimated to be about 0.9 amino acid substitutions per
site every billion years. By comparing the human α- and
b-globins, the average number of amino acid substitu-
tions per site has been estimated to be 0.800. From this
estimate, calculate when the duplication event that
produced the α- and b-globin genes must have occurred.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 94 8/14/2015 6:43:07 PM

Answers to All Questions and Problems WC-95
ANS: The total elapsed evolutionary time is 0.800/0.9 880
million years, which must be apportioned equally to the
and gene lineages by dividing by 2; thus, the time
since the duplication event is estimated to be 440 million
years.
24.13 Ribonuclease, a protein that degrades RNA, is 124 amino
acids long. A comparison between the amino acid
sequences of cow and rat ribonucleases reveals 40 differ-
ences. What is the average number of amino acid substi-
tutions that have occurred per site in these two
evolutionary lineages? If the cow and the rat lineages
diverged from a common ancestor 80 million years ago,
what is the rate of ribonuclease evolution?
ANS: Estimate the average number of substitutions per site in
the ribonuclease molecule as ln(S), where S (124
40)/124 0.68, the proportion of amino acids that are
the same in the rat and cow molecules. The average
number of substitutions per site since the cow and rat
lineages diverged from a common ancestor is therefore
0.39. The evolutionary rate in the cow and rat lineages is
0.39/(2 80 million years) 2.4 substitutions per site
every billion years.
24.14 If a randomly mating population is segregating n selec-
tively neutral alleles of a gene and each allele has the
same frequency, what is the frequency of all the homozy-
gotes in the population?
ANS: With n alleles having equal frequency, the frequency of
any one allele is 1/n. Under random mating, the fre-
quency of homozygotes for a particular allele is (1/n)
2
.
The frequency of all the homozygotes is therefore
∑(1/n)
2
n(1/n)
2
1/n.
24.15 If the evolutionary rate of amino acid substitution in a
protein is K, what is the average length of time between
successive amino acid substitutions in this protein?
ANS: The reciprocal of the rate, that is, 1/K.
24.16 The coding sequence of the alcohol dehydrogenase
(Adh) gene of D. melanogaster consists of 765 nucleo-
tides (255 codons); 192 of these nucleotides are func-
tionally silent—that is, they can be changed without
changing an amino acid in the Adh polypeptide. In a
study of genetic variation in the Adh gene, Martin
Kreitman observed that 13 of the 192 silent nucleo-
tides were polymorphic. If the same level of polymor-
phisms existed among the nonsilent nucleotides of the
Adh gene, how many amino acid polymorphisms
would Kreitman have observed in the populations he
studied?
ANS: The fraction of polymorphic sites among the silent
nucleotides is 13/192 0.068. If the same level of poly-
morphism existed among the nonsilent sites, the number
of amino acid polymorphisms would be 225 0.067
17.2. Kreitman actually observed only one amino acid
polymorphism—evidence that amino acid changes in
alcohol dehydrogenase are deleterious.
24.17 How might you explain the 1000-fold difference in the
evolutionary rates of brinopeptide and histone 3?
ANS: The protein with the higher evolutionary rate is not as
constrained by natural selection as the protein with the
lower evolutionary rate.
24.18 A geneticist has studied the sequence of a gene in each of
three species, A, B, and C. Species A and species B are
sister species; species C is more distantly related. The
geneticist has calculated the ratio of nonsynonymous
(NS) to synonymous (S) nucleotide substitutions in the
coding region of the gene in two ways—rst, by compar-
ing the gene sequences of species A and C, and second,
by comparing the gene sequences of species B and C.
The NS:S ratio for the comparison of species B and C is
ve times greater than it is for the comparison of species
A and C. What might this difference in the NS:S ratios
suggest?
ANS: The difference in the NS:S ratios suggests that in at least
one lineage, positive selection has been operating to
change nucleotides in the gene.
24.19 Dispersed, repetitive sequences such as transposable ele-
ments may have played a role in duplicating short regions
in a genome. Can you suggest a mechanism? (Hint: See
Chapter 21 on the Instructor Companion site.)
ANS: Repetitive sequences that are near each other can medi-
ate displaced pairing during meiosis. Exchange involving
the displaced sequences can duplicate the region between
them.
24.20 Exon shufing is a mechanism that combines exons from
different sources into a coherent sequence that can
encode a composite protein—one that contains peptides
from each of the contributing exons. Alternate splicing is
a mechanism that allows exons to be deleted during the
expression of a gene; the mRNAs produced by alternate
splicing may encode different, but related, polypeptides
(see Chapter 18). What bearing do these two mecha-
nisms have on the number of genes in a eukaryotic
genome? Do these mechanisms help to explain why the
gene number in the nematode Caenorabditis elegans is not
too different from the gene number in Homo sapiens?
ANS: Exon shufing is a way of creating genes that have pieces
from disparate sources. Through exon shufing, the
number of genes in a genome could be increased without
having to evolve the genes “from scratch.” However, the
genome sequencing projects indicate that the gene num-
ber in complex, multicellular vertebrates is not much dif-
ferent from the gene number in simple, multicellular
invertebrates or in plants. If, judging from their pheno-
types, multicellular vertebrates need more gene products
than phenotypically simpler invertebrates such as C. ele-
gans, alternate splicing could provide some of these gene
products without increasing the number of genes.
24.21 Drosophila mauritiana inhabits the island of Mauritius in
the Indian Ocean. Drosophila simulans, a close relative, is
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 95 8/14/2015 6:43:07 PM

96-WC Answers to All Questions and Problems
widely distributed throughout the world. What experi-
mental tests would you perform to determine if D. mau-
ritiana and D. simulans are genetically different species?
ANS: Cross D. mauritiana with D. simulans and determine if
these two species are reproductively isolated. For
instance, can they produce offspring? If they can, are the
offspring fertile?
24.22 Distinguish between allopatric and sympatric modes of
speciation.
ANS: Allopatric speciation occurs when populations diverge
genetically while they are geographically separated.
Sympatric speciation occurs when populations diverge
genetically while they inhabit the same territory.
24.23 The prune gene (symbol pn) is X-linked in Drosophila
melanogaster. Mutant alleles of this gene cause the eyes to
be brown instead of red. A dominant mutant allele of
another gene located on a large autosome causes
hemizygous or homozygous pn ies to die; this dominant
mutant allele is therefore called Killer of prune (symbol
Kpn). How could mutants such as these play a role in
the evolution of reproductive isolation between
populations?
ANS: The Kpn–pn interaction is an example of the kind of neg-
ative epistasis that might prevent populations that have
evolved separately from merging into one panmictic
population. The Kpn mutation would have evolved in
one population and the pn mutation in another, geo-
graphically separate population. When the populations
merge, the two mutations can be brought into the same
y by interbreeding. If the combination of these muta-
tions is lethal, then the previously separate populations
will not be able to exchange genes, that is, they will be
reproductively isolated.
24.24 A segment of DNA in an individual may differ at several
nucleotide positions from a corresponding DNA seg-
ment in another individual. For instance, one individual
may have the sequence …A…G…C… and another indi-
vidual may have the sequence …T…A…A…. These two
DNA segments differ in three nucleotide positions.
Because the nucleotides within each segment are tightly
linked, they will tend to be inherited together as a unit,
that is, without being scrambled by recombination.
We call such heritable units DNA haplotypes. Through
sampling and DNA sequencing, researchers can deter-
mine which DNA haplotypes are present in a particular
population. When this kind of analysis is performed on
human populations by sequencing, for example, a seg-
ment of mitochondrial DNA, it is found that samples
from Africa exhibit more haplotype diversity than sam-
ples from other continents. What does this observation
tell us about human evolution?
ANS: More haplotype diversity refers to the number of differ-
ent haplotypes that are found in a population. If African
populations of humans have the greatest haplotype
diversity, then these populations appear to have had a
longer time to accumulate different haplotypes—that is,
they are older than other populations. Greater haplotype
diversity in African populations of humans is therefore
evidence that African populations were at the root of the
modern human evolutionary tree.
ONLINE_AnswerstoOddNumberedQuestionsandProblems.indd 96 8/14/2015 6:43:07 PM
